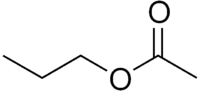
Propyl acetate
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Propyl ethanoate | |
| Other names
Propyl acetate; n-Propyl ethanoate; n-Propyl acetate; Propylacetate; Acetic acid, propyl ester; n-Propyl ester of acetic acid | |
| Identifiers | |
| 109-60-4 | |
| ChEBI | CHEBI:40116 |
| ChEMBL | ChEMBL44857 |
| ChemSpider | 7706 |
| DrugBank | DB01670 |
| |
| Jmol-3D images | Image |
| PubChem | 7997 |
| |
| UNII | 4AWM8C91G6 |
| Properties | |
| Molecular formula |
C5H10O2 |
| Molar mass | 102.13 g·mol−1 |
| Appearance | Clear, colorless liquid |
| Density | 0.89 g/cm3[1] |
| Melting point | −95 °C (−139 °F; 178 K)[1] |
| Boiling point | 102 °C (216 °F; 375 K)[1] |
| 18.9 g/L[1] | |
| Hazards | |
| EU classification | Flammable (F) Irritant (Xi) |
| R-phrases | R11, R36 |
| S-phrases | (S2), S16, S26, S29, S33 |
| NFPA 704 | |
| Flash point | 10 °C (50 °F; 283 K)[1] |
| 450 °C (842 °F; 723 K) | |
| LD50 (Median lethal dose) |
9370 mg/kg (oral, rat)[2] 17800 mg/kg (dermal, rabbit)[3] |
| Related compounds | |
| Related esters |
ethyl acetate n-butyl acetate isobutyl acetate |
| Related compounds |
propan-1-ol acetic acid |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Propyl acetate, also known as propyl ethanoate, is a chemical compound used as a solvent. This clear, colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and 1-propanol, often via Fischer–Speier esterification.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Record in the GESTIS Substance Database of the IFA
- ↑ Jenner, P; Hagan, E; Taylor, J; Cook, E; Fitzhugh, O (1964). "Food flavourings and compounds of related structure I. Acute oral toxicity". Food and Cosmetics Toxicology 2: 327. doi:10.1016/S0015-6264(64)80192-9.
- ↑ Union Carbide Data Sheet. Vol. 1/25/1965
External links
- NIOSH Pocket Guide to Chemical Hazards
- Acetic acid, propyl ester - Toxicity Data
- N-Propyl Acetate MSDS
