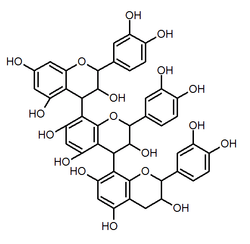
Procyanidin C2
 | |
| Names | |
|---|---|
| Other names
C-(4,8)-C-(4,8)-C Procyanidin trimer C2 Catechin-(4alpha→8)-Catechin-(4alpha→8)-Catechin Catechin-(4α→8)-catechin-(4α→8)-catechin Trimer C2 | |
| Identifiers | |
| ChEBI | CHEBI:75644 |
| |
| Jmol-3D images | Image |
| |
| Properties | |
| C45H38O18 | |
| Molar mass | 866.74 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.
Natural occurrences
Procyanidin C2 is found in grape seeds (Vitis vinifera)[1][2] and wine,[3] in barley (Hordeum vulgare),[4] malt[5] and beer,[6] in Betula spp., in Pinus radiata, in Potentilla viscosa, in Salix caprea or in Cryptomeria japonica.[7][8][9]
The contents in barley grain of trimeric proanthocyanidins, including procyanidin C2, range from 53 to 151 μg catechin equivalents/g.[10]
Possible health uses
Proanythocyanidin oligomers, extracted from grape seeds, have been used for the experimental treatment of androgenic alopecia. When applied topically, they promote hair growth in vitro, and induce anagen in vivo. Procyanidin C2 is the subtype of extract most effective.[11]
Experiments showed that both procyanidin C2 and Pycnogenol (French maritime pine bark extract) increase TNF-α secretion in a concentration- and time-dependent manner. These results demonstrate that procyanidins act as modulators of the immune response in macrophages.[12]
Chemistry
In the presence of procyanidin C2, the red color of the anthocyanin oenin appears more stable. However, the HPLC chromatogram shows a decrease in the amplitude of the peaks of oenin and procyanidin C2. Concomitantly, a new peak appears with a maximal absorption in the red region. This newly formed pigment probably comes from the condensation of oenin and procyanidin C2.[13]
Chemical synthesis
A stereoselective synthesis of benzylated catechin trimer under intermolecular condensasion is achieved using equimolar amount of dimeric catechin nucleophile and monomeric catechin electrophile catalyzed by AgOTf or AgBF4. The coupled product can be transformed into procyanidin C2 by a known procedure.[14]
The stereoselective synthesis of seven benzylated proanthocyanidin trimers (epicatechin-(4β-8)-epicatechin-(4β-8)-epicatechin trimer (procyanidin C1), catechin-(4α-8)-catechin-(4α-8)-catechin trimer (procyanidin C2), epicatechin-(4β-8)-epicatechin-(4β-8)-catechin trimer and epicatechin-(4β-8)-catechin-(4α-8)-epicatechin trimer derivatives) can be achieved with TMSOTf-catalyzed condensation reaction, in excellent yields. The structure of benzylated procyanidin C2 was confirmed by comparing the 1H NMR spectra of protected procyanidin C2 that was synthesized by two different condensation approaches. Finally, deprotection of (+)-catechin and (−)-epicatechin trimers derivatives gives four natural procyanidin trimers in good yields.[15]
Molar equivalents of synthetic (2R,3S,4R or S)-leucocyanidin and (+)-catechin condense with exceptional rapidity at pH 5 under ambient conditions to give the all-trans-[4,8]- and [4,6]-bi-[(+)-catechins] (procyanidins B3, B6) the all-trans-[4,8:4,8]- and [4,8:4,6]-tri-[(+)-catechins] (procyanidin C2 and isomer).[16]
Iterative oligomer chemical synthesis
A coupling utilising a C8-boronic acid as a directing group was developed in the synthesis of natural procyanidin B3 (i.e., 3,4-trans-(+)-catechin-4α→8-(+)-catechin dimer). The key interflavan bond is forged using a Lewis acid-promoted coupling of C4-ether with C8-boronic acid to provide the α-linked dimer with high diastereoselectivity. Through the use of a boron protecting group, the coupling procedure can be extended to the synthesis of a protected procyanidin trimer analogous to natural procyanidin C2.[17]
See also
References
- ↑ Romeyer, F. M.; Macheix, J. J.; Sapis, J. C. (1985). "Changes and importance of oligomeric procyanidins during maturation of grape seeds". Phytochemistry 25: 219. doi:10.1016/S0031-9422(00)94532-1.
- ↑ Tsang, C.; Auger, C.; Mullen, W.; Bornet, A.; Rouanet, J. M.; Crozier, A.; Teissedre, P. L. (2005). "The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats". The British journal of nutrition 94 (2): 170–181. doi:10.1079/BJN20051480. PMID 16115350.
- ↑ Identification of the condensed tannins content in grape and Bordeaux wine by means of standards of synthesis. S. Fabre, E. Fouquet, I. Pianet and P-L. Teissedre (article)
- ↑ Kristiansen, K. N. (1984). "Biosynthesis of proanthocyanidins in barley: Genetic control of the conversion of dihydroquercetin to catechin and procyanidins". Carlsberg Research Communications 49 (5): 503–524. doi:10.1007/BF02907552.
- ↑ Goupy, Pascale; Hugues, Mireille; Boivin, Patrick; Amiot, Marie Josèphe (1999). "Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds". Journal of the Science of Food and Agriculture 79 (12): 1625. doi:10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8.
- ↑ McMurrough, I.; Madigan, D.; Smyth, M. R. (1996). "Semipreparative Chromatographic Procedure for the Isolation of Dimeric and Trimeric Proanthocyanidins from Barley". Journal of Agricultural and Food Chemistry 44 (7): 1731. doi:10.1021/jf960139m.
- ↑ Harborne, The Handbook of Natural Flavonoids, 2, 1999, page 355, Flavans and proanthocyanidins, ISBN 047195893X
- ↑ Thompson, R. S.; Jacques, D.; Haslam, E.; Tanner, R. J. N. (1972). "Plant proanthocyanidins. Part I. Introduction; the isolation, structure, and distribution in nature of plant procyanidins". Journal of the Chemical Society, Perkin Transactions 1: 1387. doi:10.1039/P19720001387.
- ↑ Brandon, M. J.; Foo, L. Y.; Porter, L. J.; Meredith, P. (1980). "Proanthocyanidins of barley and sorghum; composition as a function of maturity of barley ears". Phytochemistry 21 (12): 2953. doi:10.1016/0031-9422(80)85076-X.
- ↑ Quinde-Axtell, Z.; Baik, B. K. (2006). "Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration". Journal of Agricultural and Food Chemistry 54 (26): 9978–9984. doi:10.1021/jf060974w. PMID 17177530.
- ↑ Takahashi, T.; Kamiya, T.; Hasegawa, A.; Yokoo, Y. (1999). "Procyanidin Oligomers Selectively and Intensively Promote Proliferation of Mouse Hair Epithelial Cells in Vitro and Activate Hair Follicle Growth in Vivo1". Journal of Investigative Dermatology 112 (3): 310–316. doi:10.1046/j.1523-1747.1999.00532.x. PMID 10084307.
- ↑ Park, Y. C.; Rimbach, G.; Saliou, C.; Valacchi, G.; Packer, L. (2000). "Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-α secretion, and NF-κB-dependent gene expression in RAW 264.7 macrophages". FEBS Letters 465 (2–3): 93–97. doi:10.1016/S0014-5793(99)01735-4. PMID 10631311.
- ↑ Malien-Aubert, C.; Dangles, O.; Amiot, M. J. (2002). "Influence of procyanidins on the color stability of oenin solutions". Journal of Agricultural and Food Chemistry 50 (11): 3299–3305. doi:10.1021/jf011392b. PMID 12010001.
- ↑ Makabe, H.; Oizumi, Y.; Mohri, Y.; Hattori, Y. (2011). "Efficient Stereoselective Synthesis of Catechin Trimer Derivative Using Silver Lewis Acid-Mediated Equimolar Condensation". Heterocycles 83 (4): 739. doi:10.3987/COM-11-12159.
- ↑ Nakajima, N.; Saito, A.; Tanaka, A.; Ubukata, M. (2004). "Efficient Stereoselective Synthesis of Proanthocyanidin Trimers with TMSOTf-Catalyzed Intermolecular Condensation". Synlett (6): 1069. doi:10.1055/s-2004-822905.
- ↑ Delcour, J. A.; Ferreira, D.; Roux, D. G. (1983). "Synthesis of condensed tannins. Part 9. The condensation sequence of leucocyanidin with (+)-catechin and with the resultant procyanidins". Journal of the Chemical Society, Perkin Transactions 1: 1711. doi:10.1039/P19830001711.
- ↑ Dennis, E. G.; Jeffery, D. W.; Johnston, M. R.; Perkins, M. V.; Smith, P. A. (2012). "Procyanidin oligomers. A new method for 4→8 interflavan bond formation using C8-boronic acids and iterative oligomer synthesis through a boron-protection strategy". Tetrahedron 68: 340. doi:10.1016/j.tet.2011.10.039. INIST:25254810.
External links
| ||||||||||||||