Procyanidin B3
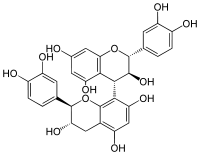 | |
| Names | |
|---|---|
| IUPAC name
(2R,2ʼR,3S,3ʼS,4S)-2,2ʼ-bis(3,4-dihydroxyphenyl)-3,3ʼ,4,4ʼ-tetrahydro-2H,2ʼH-4,8ʼ-bichromene-3,3ʼ,5,5ʼ,7,7ʼ-hexol | |
| Other names
Procyanidin B3 | |
| Identifiers | |
| ChEBI | CHEBI:75630 |
| Jmol-3D images | Image |
| PubChem | 146798 |
| |
| Properties | |
| C30H26O12 | |
| Molar mass | 578.52 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Procyanidin B3 is a B type proanthocyanidin. Procyanidin B3 is a catechin dimer (catechin-(4α→8)-catechin).
Natural occurrences
It can be found in red wine,[1] in barley,[2][3] in beer,[4] in peach[5] or in Jatropha macrantha, the Huanarpo Macho.[6]
Health effects
It has been identified as an hair-growth stimulant.[2]
Chemical synthesis
Molar equivalents of synthetic (2R,3S,4R or S)-leucocyanidin and (+)-catechin condense with exceptional rapidity at pH 5 under ambient conditions to give the all-trans-[4,8]- and [4,6]-bi-[(+)-catechins] (procyanidins B3, B6) the all-trans-[4,8:4,8]- and [4,8:4,6]-tri-[(+)-catechins] (procyanidin C2 and isomer).[7]
See also
References
- ↑ C. Dallas, J.M. Ricardo-Da-Silva and Olga Laureano (1995). "Degradation of oligomeric procyanidins and anthocyanins in a Tinta Roriz red wine during maturation" (PDF). Vitis 34 (1): 51–56.
- ↑ 2.0 2.1 Kamimura, A; Takahashi, T (2002). "Procyanidin B-3, isolated from barley and identified as a hair-growth stimulant, has the potential to counteract inhibitory regulation by TGF-beta1". Experimental dermatology 11 (6): 532–41. doi:10.1034/j.1600-0625.2002.110606.x. PMID 12473061.
- ↑ Quinde-Axtell, Zory; Baik, Byung-Kee (2006). "Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration". Journal of Agricultural and Food Chemistry 54 (26): 9978–84. doi:10.1021/jf060974w. PMID 17177530.
- ↑ Delcour, Jan (1985). Structure elucidation of proanthocyanidins: Direct synthesis and isolation from Pilsener beer.
- ↑ Infante, Rodrigo; Contador, Loreto; Rubio, Pía; Aros, Danilo; Peña-Neira, Álvaro (2011). "Postharvest Sensory and Phenolic Characterization of Elegant Lady and Carson Peaches" (PDF). Chilean journal of agricultural research 71 (3): 445. doi:10.4067/S0718-58392011000300016.
- ↑ Benavides, Angelyne; Montoro, Paola; Bassarello, Carla; Piacente, Sonia; Pizza, Cosimo (2006). "Catechin derivatives in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali–quantitative analysis". Journal of Pharmaceutical and Biomedical Analysis 40 (3): 639–47. doi:10.1016/j.jpba.2005.10.004. PMID 16300918.
- ↑ Delcour, Jan. A.; Ferreira, Daneel; Roux, David G. (1983). "Synthesis of condensed tannins. Part 9. The condensation sequence of leucocyanidin with (+)-catechin and with the resultant procyanidins". Journal of the Chemical Society, Perkin Transactions 1: 1711. doi:10.1039/P19830001711.
| ||||||||||||||