Procyanidin B2
 | |
| Names | |
|---|---|
| IUPAC name
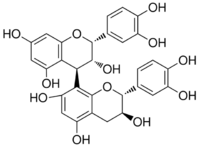
(2R,2ʼR,3R,3ʼR,4R)-2,2ʼ-bis(3,4-dihydroxyphenyl)-3,3ʼ,4,4ʼ-tetrahydro-2H,2ʼH-4,8ʼ-bichromene-3,3ʼ,5,5ʼ,7,7ʼ-hexol | |
| Other names
Procyanidin-B2 (−)-Epicatechin-(4β→8)-(−)-epicatechin | |
| Identifiers | |
| 29106-49-8 | |
| ChEBI | CHEBI:75632 |
| Jmol-3D images | Image |
| PubChem | 122738 |
| |
| Properties | |
| C30H26O12 | |
| Molar mass | 578.52 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Procyanidin B2 is a B type proanthocyanidin. Its structure is (−)-Epicatechin-(4β→8)-(−)-epicatechin.
Procyanidin B2 can be found in Cinchona pubescens (Chinchona, in the rind, bark and cortex), in Cinnamomum verum (Ceylon cinnamon, in the rind, bark and cortex), in Crataegus monogyna (Common hawthorn, in the flower and blossom), in Uncaria guianensis (Cat's claw, in the root), in Vitis vinifera (Common grape vine, in the leaf),[1] in Litchi chinensis (litchi, in the pericarp),[2] in the apple,[3] and in Ecdysanthera utilis.[4]
Procyanidin B2 can be converted into procyanidin A2 by radical oxidation using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals under neutral conditions.[5]
Procyanidin B2 has been shown to inhibit the formation of the advanced glycation end-products pentosidine, carboxymethyllysine (CML), and methylglyoxal (MGO).[6]
See also
References
- ↑ Proanthocyanidin-B2 on liberherbarum.com
- ↑ Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Mouming Zhao; Bao Yang; Jinshui Wang; Yang Liu; Limei Yu; Yueming Jiang, 2007
- ↑ Proanthocyanidin-B2 on fuzing.com
- ↑ Immunomodulatory Proanthocyanidins from Ecdysanthera utilis. Lie-Chwen Lin, Yuh-Chi Kuo, and Cheng-Jen Chou, 2002
- ↑ Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Kazunari Kondo, Masaaki Kurihara, Kiyoshi Fukuhara, Takashi Tanaka, Takashi Suzuki, Naoki Miyata and Masatake Toyoda, Tetrahedron Letters, 22 January 2000, Volume 41, Issue 4, Pages 485–488, doi:10.1016/S0040-4039(99)02097-3
- ↑ Peng X, Ma J, Chao J, Sun Z, Chang RC, Tse I, Li ET, Chen F, Wang M (2010). "Beneficial effects of cinnamon proanthocyanidins on the formation of specific advanced glycation endproducts and methylglyoxal-induced impairment on glucose consumption". Journal of Agricultural and Food Chemistry 58 (11): 6692–6696. doi:10.1021/jf100538t. PMID 20476737.
| ||||||||||||||