Praseodymium(III) oxide
 | |
| Names | |
|---|---|
| IUPAC name
Praseodymium(III) oxide | |
| Other names
Praseodymium oxide, Praseodymium sesquioxide | |
| Identifiers | |
| 11113-81-8 | |
| EC number | 234-845-3 |
| PubChem | 165911 |
| Properties | |
| Pr2O3 | |
| Molar mass | 329.813 g/mol |
| Appearance | white hexagonal crystals |
| Density | 6.9 g/cm3 |
| Melting point | 2,183 °C (3,961 °F; 2,456 K) |
| Boiling point | 3,760 °C (6,800 °F; 4,030 K)[1] |
| Structure | |
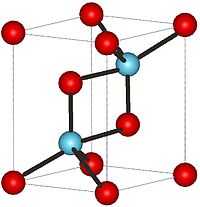
| Crystal structure | Hexagonal, hP5 |
| Space group | P-3m1, No. 164 |
| Thermochemistry | |
| Specific heat capacity (C) |
117.4 J•mol−1•K−1[1] |
| Std enthalpy of formation (ΔfH |
-1809.6 kJ•mol−1 |
| Related compounds | |
| Other anions |
Praseodymium(III) chloride Praseodymium(III) sulfide |
| Other cations |
Neodymium(III) oxide Promethium(III) oxide Cerium(III) oxide |
| Related compounds |
Uranium(VI) oxide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Praseodymium(III) oxide or praseodymium oxide is the chemical compound composed of praseodymium and oxygen with the formula Pr2O3. It forms white hexagonal crystals.[1] Praseodymium(III) oxide crystallizes in the manganese(III) oxide or bixbyite structure.[2]
Uses
Praseodymium(III) oxide can be used as a dielectric in combination with silicon.[2] Praseodymium-doped glass, called didymium glass, turns yellow and is used in welding goggles because it blocks infrared radiation. 2500 tonnes of praseodymium(III) oxide are produced worldwide each year.[3] Praseodymium(III) oxide is also used to color glass and ceramics yellow.[4] For coloring ceramics, also the very dark brown mixed-valence compound praseodymium(III,IV)oxide, Pr6O11, is used.
References
- ↑ 1.0 1.1 1.2 Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 478, 523, ISBN 0-8493-0594-2
- ↑ 2.0 2.1 Dabrowski, Jarek; Weber, Eicke R. (2004), Predictive Simulation of Semiconductor Processing, Springer, p. 264, ISBN 978-3-540-20481-7, retrieved 2009-03-18
- ↑ Emsley, John (2003), Nature's Building Blocks, Oxford University Press, p. 341, ISBN 978-0-19-850340-8, retrieved 2009-03-18
- ↑ Krebs, Robert E. (2006), The History and Use of our Earth's Chemical Elements, Greenwood Publishing Group, p. 283, ISBN 978-0-313-33438-2, retrieved 2009-03-18
| ||||||