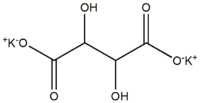
Potassium tartrate
 | |
| Names | |
|---|---|
| IUPAC name
Dipotassium 2,3-dihydroxybutanedioate | |
| Other names
Dipotassium tartrate; Argol; E336 | |
| Identifiers | |
| 921-53-9 (L) | |
| ChemSpider | 2697916 |
| |
| Jmol-3D images | Image |
| PubChem | 8984 |
| |
| Properties | |
| C4H4K2O6 | |
| Molar mass | 226.268 g/mol |
| Appearance | colorless, slightly opaque crystals |
| Density | 1.984 g/cm3 |
| Solubility | insoluble in alcohol |
| Refractive index (nD) |
1.550 |
| Structure | |
| Crystal structure | monoclinic |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Potassium tartrate, dipotassium tartrate or argol has formula K2C4H4O6. It is the potassium salt of tartaric acid. It is often confused with potassium bitartrate, also known as cream of tartar. As a food additive, it shares the E number E336 with potassium bitartrate.
Manufacturing
Potassium tartrate is produced by the reaction of tartaric acid with potassium sodium tartrate (rochelle salt), and potassium sulfate, followed by filtration, purification, precipitation and drying.
Other compounds
Tartar emetic is produced when potassium tartrate is heated with antimony trioxide. Tartar emetic causes intense nausea, prostration and vomiting by irritating the gastrointestinal mucosa.