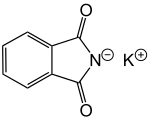
Potassium phthalimide
 | |
| Identifiers | |
|---|---|
| 1074-82-4 | |
| ChemSpider | 10627162 |
| |
| Jmol-3D images | Image |
| PubChem | 3356745 |
| |
| Properties | |
| C8H4KNO2 | |
| Molar mass | 185.221 g/mol |
| Appearance | Light yellow solid |
| Melting point | > 300 °C |
| Soluble in water, | |
| Hazards | |
| NFPA 704 | |
| Related compounds | |
| Related compounds |
Phthalimide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Potassium phthalimide is a chemical compound of formula C8H4KNO2. It is commercially available, and usually presents as fluffy, very pale yellow crystals. It is the potassium salt of phthalimide. If desired, it may be prepared by adding a hot solution of phthalimide to a solution of potassium hydroxide; the desired product precipitates.[1]
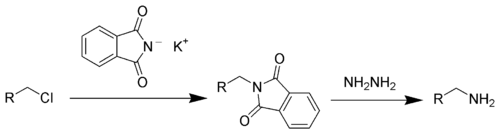
This compound is a reagent used in the Gabriel synthesis of amines.
References
- ↑ P. L. Salzberg and J. V. Supniewski (1941). "β-Bromoethylphthalimide". Org. Synth.; Coll. Vol. 1, p. 119