Polystyrene sulfonate
 | |
| Systematic (IUPAC) name | |
|---|---|
| Poly(4-vinylbenzenesulfonic acid) | |
| Clinical data | |
| Trade names |
Sodium salt: Kayexalate, Kionex, Resonium A, sodium polystyrene sulfonate Calcium salt: Calcium Resonium, Sorbisterit, Resikali, Calcium Resonium, Sorbisterit, Resikali |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682108 |
| |
| |
| Oral, retention enema | |
| Pharmacokinetic data | |
| Bioavailability | None |
| Metabolism | None |
| Excretion | Faeces (100%) |
| Identifiers | |
|
28210-41-5 | |
| V03AE01 | |
| PubChem | CID 75905 |
| DrugBank |
DB01344 |
| Chemical data | |
| Formula | [C8H8SO3]n |
| | |
Polystyrene sulfonates are polymers derived from polystyrene but containing sulfonic acid or sulfonate functional groups. The linear polymer is white when very pure and is water-soluble. The crosslinked material (called a resin) does not dissolve in water and typically appears amber in color. These polymers are classified as polysalts and ionomers. They are widely used to remove ions from a solution in technical or medical applications.[1]
Production and chemical structure
Polystyrene sulfonic acid has the idealized formula (CH2CHC6H4SO3H)n. The material is prepared by sulfonation of polystyrene:
- (CH2CHC6H5)n + n SO3 → (CH2CHC6H4SO3H)n
Several methods exist for this conversion, which can lead to varying degree of sulfonation. Usually the polystyrene is crosslinked, which keeps the polymer from dissolving. Since the sulfonic acid group (SO3H) is strongly acidic, this polymer neutralizes bases. In this way, various salts of the polymer can be prepared, leading to sodium, calcium, and other salts:
- (CH2CHC6H4SO3H)n + n NaOH → (CH2CHC6H4SO3Na)n + n H2O
These ion-containing polymers are called ionomers.
Alternative sulfonation methods
Double substitutions of the phenyl rings are known to occur, even with conversions well below 100%. Crosslinking reactions are also found, where condensation of two sulfonic acid groups yields a sulfonyl crosslink. On the other hand, the use of milder conditions such as acetyl sulfate leads to incomplete sulfonation. Recently, the atom transfer radical polymerization (ATRP) of protected styrenesulfonates has been reported,[2][3] leading to well defined linear polymers, as well as more complicated molecular architectures.[4]
Applications
Polystyrene sulfonates are useful because of their ion exchange properties.[1]
Water softening
Water softening is achieved by percolating hard water through a bed of sodium form of cross-linked polystyrene sulfonate. The hard ions such as calcium (Ca2+) and magnesium (Mg2+) adhere to the sulfonate groups, displacing sodium ions. The resulting solution of sodium ions is softened.
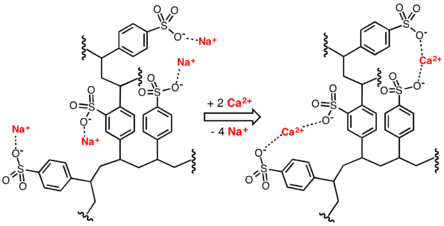
Medical applications
For medical applications, polystyrene sulfonate is usually supplied in the sodium and calcium form. Its use is indicated as potassium binders in acute and chronic kidney disease for patients suffering from hyperkalaemia (abnormal high blood serum potassium levels), which may, e.g., result in weakness or irregular heartbeat.[5][6] Excess blood potassium also arises due to digoxin toxicity,[7] although there is concern about possible side effects when it is mixed with sorbitol.[8]
Polystyrene sulfonates are administered orally (with a meal) or rectally, by retention enema.[9] In the large intestines, potassium derived from food is exchanged for sodium or calcium ions, respectively.[10] Finally, the indigestible potassium polystryene sulfonate complex is excreted with the feces, preventing the absorption of potassium into the blood stream. Hence, the serum potassium level decreases.
Side-effects
Intestinal disturbances are common, including loss of appetite, nausea, vomiting, and constipation. In rare cases, it has been associated with colonic necrosis.[11] Changes in electrolyte blood levels may occur such as hypermagnesemia, hypercalcemia, and hypokalemia.[5]
Other uses
Sodium polystyrene sulfonate is used as a superplastifier in cement, as a dye improving agent for cotton, and as proton exchange membranes in fuel cell applications. In their acid form, the resin is used as a solid acid catalyst in organic synthesis.[12]
References
- ↑ 1.0 1.1 François Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_393.pub2
- ↑ Sikkema, FD; Comellas-Aragonès, M; Fokkink, RG; Verduin, BJ; Cornelissen, JJ; Nolte, RJ (2007). "Monodisperse polymer-virus hybrid nanoparticles". Organic & biomolecular chemistry 5 (1): 54–7. doi:10.1039/b613890j. PMID 17164905.
- ↑ Lienkamp, Karen; Schnell, Ingo; Groehn, Franziska; Wegner, Gerhard (2006). "Polymerization of Styrene Sulfonate Ethyl Ester by ATRP: Synthesis and Characterization of Macromonomers for Suzuki Polycondensation". Macromolecular Chemistry and Physics 207 (22): 2066. doi:10.1002/macp.200600322.
- ↑ Lienkamp, Karen; Ruthard, Christian; Lieser, GüNter; Berger, RüDiger; Groehn, Franziska; Wegner, Gerhard (2006). "Polymerization of Styrene Sulfonate Ethyl Ester and Styrene Sulfonate Dodecyl Ester by ATRP: Synthesis and Characterization of Polymer Brushes". Macromolecular Chemistry and Physics 207 (22): 2050. doi:10.1002/macp.200600321.
- ↑ 5.0 5.1 Sorbisterit – Summary of Product Characteristics; Retrieved: 27 November 2009.
- ↑ U.S. National Library of Medicine, National Institutes of Health, URL: http://www.nlm.nih.gov/medlineplus/ency/article/001179.htm; Retrieved: 27 November 2009
- ↑ Watson, M; Abbott, KC; Yuan, CM (2010). "Damned if you do, damned if you don't: potassium binding resins in hyperkalemia.". Clinical journal of the American Society of Nephrology : CJASN 5 (10): 1723–6. doi:10.2215/CJN.03700410. PMID 20798253.
- ↑ Sterns RH, Rojas M, Bernstein P, Chennupati S (May 2010). "Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective?". J. Am. Soc. Nephrol. 21 (5): 733–5. doi:10.1681/ASN.2010010079. PMID 20167700.
- ↑ Medicines Complete: Martindale: The Complete Drug Reference, URL: http://www.medicinescomplete.com/mc/martindale/2009/5004-l.htm; Retrieved: 27 November 2009
- ↑ Lederer, E., Ouseph, R., Nayak, V., Dinh, S., URL: http://emedicine.medscape.com/article/240903-treatment; Retrieved: 27 November 2009
- ↑ Rogers FB, Li SC (August 2001). "Acute colonic necrosis associated with sodium polystyrene sulfonate (Kayexalate) enemas in a critically ill patient: case report and review of the literature". J Trauma 51 (2): 395–7. doi:10.1097/00005373-200108000-00031. PMID 11493807.
- ↑ Erik Gálvez, Pedro Romea, and Fèlix Urpí (2009). "Stereoselective Synthesis of anti α-Methyl-β-Methoxy Carboxylic Compounds". Org. Synth. 86: 81.
External links
- Potassium and your CKD Diet - kidney.org
- Information on Hyperkalaemia by Fresenius Medical Care - fmc-renalpharma.com
| ||||||||||||||||||||||||||||