Platinum pentafluoride
 | |
| Names | |
|---|---|
| IUPAC name
platinum(V) fluoride | |
| Identifiers | |
| 13782-84-8 | |
| Properties | |
| F5Pt | |
| Appearance | red solid |
| Melting point | 75-6 °C |
| Boiling point | 300-5 °C |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Platinum pentafluoride is the inorganic compound with the empirical formula PtF5. This red volatile solid has rarely been studied but is of interest as a binary fluoride of platinum, i.e. a compound containing only Pt and F. It is hydrolyzed in water.[1]
The compound was first prepared by Neil Bartlett by fluorination of platinum dichloride above 350 °C (below that temperature, only PtF4 forms).[1]
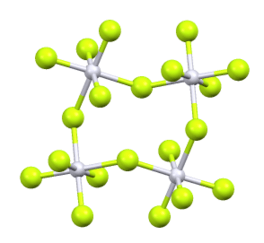
Its structure consists of a tetramer]], very similar to that of ruthenium pentafluoride. Within the tetramers, each Pt adopts octahedral molecular geometry, with two bridging fluoride ligands.[2]
References
- ↑ 1.0 1.1 Bartlett, N.; Lohmann, D. H. "Two New Fluorides of Platinum" Proceedings of the Chemical Society, London 1960, pp. 14-15.
- ↑ Mueller, B. G.; Serafin, M. (1992). "Single-crystal investigations on PtF4 and PtF5". European Journal of Solid State Inorganic Chemistry 29: 625–633. doi:10.1002/chin.199245006.
| ||||||||||||||||||||||||||||