Plasmodium ovale
| Plasmodium ovale | |
|---|---|
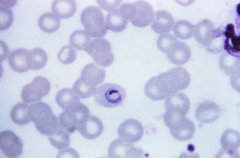 | |
| Plasmodium ovale trophozoite, Giemsa stain. | |
| Scientific classification | |
| Kingdom: | Protista |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Haemosporida |
| Family: | Plasmodiidae |
| Genus: | Plasmodium |
| Species: | P. ovale |
| Binomial name | |
| Plasmodium ovale Stephens 1922 | |
Plasmodium ovale is a species of parasitic protozoa that causes tertian malaria in humans. It is one of several species of Plasmodium parasites that infect humans including Plasmodium falciparum and Plasmodium vivax which are responsible for most malarial infection. It is rare compared to these two parasites, and substantially less dangerous than P. falciparum.
P. ovale has recently been shown by genetic methods to consist of two subspecies, P. ovale curtisi and P. ovale wallikeri.[1]
History
This species was first described by Stephens in a patient from East Africa in 1922.[2]
Epidemiology
While it is frequently said that P. ovale is very limited in its range being limited to West Africa,[3][4] the Philippines, eastern Indonesia, and Papua New Guinea,[5] it has been reported from Bangladesh ,[6] Cambodia,[7] India,[8] Thailand[9] and Vietnam[10]
The reported prevalence is low (<5%) with the exception of West Africa, where prevalences above 10% have been observed.
The epidemiology of this parasite is in need of updating because the most recent global map of its distribution was produced in 1969.[11]
It has been estimated that there are about 15 million cases of infection each year with this parasite.[12]
Clinical features
The prepatent period in the human ranges from 12 to 20 days. Some forms in the liver have delayed development and relapse may occur after periods of up to 4 years after infection.
The developmental cycle in the blood lasts approximately 49 h. An examination of records from induced infections indicated that there were an average of 10.3 fever episodes of > or = 101 ºF (38,3 ºC) and 4.5 fever episodes of > or = 104 ºF (40,0 ºC). Mean maximum parasite levels were 6,944/microl for sporozoite-induced infections and 7,310/microl for trophozoite-induced infections.
Diagnosis
The microscopic appearance of P. ovale is very similar to that of P. vivax and if there are only a small number of parasites seen, it may be impossible to distinguish the two species on morphological grounds alone. There is no difference between the medical treatment of P. ovale and P. vivax, and therefore some laboratory diagnoses report "P. vivax/ovale", which is perfectly acceptable as treatment for the two are very similar. Schüffner's dots are seen on the surface of the parasitised red blood cell, but these are larger and darker than in P. vivax and are sometimes called James' dots or James' stippling. About twenty percent of the parasitised cells are oval in shape (hence the species name) and some of the oval cells also have fimbriated edges (the so-called "comet cell"). The mature schizonts of P. ovale never have more than twelve nuclei within them and this is the only reliable way of distinguishing between the two species.
P. vivax and P. ovale that has been sitting in EDTA for more than half-an-hour before the blood film is made will look very similar in appearance to P. malariae, which is an important reason to warn the laboratory immediately when the blood sample is drawn so they can process the sample as soon as it arrives.
Molecular tests (tests that look for DNA material from P. ovale in blood) must take into account the fact that there are two subspecies of ovale and tests designed for one subspecies may not necessarily detect the other.[13]
Treatment
Standard treatment is concurrent treatment with chloroquine and primaquine. The combination atovaquone-proguanil may be used in those patients who are unable to take chloroquine for whatever reason.[14]
Phylogenetics
Among the species infecting the great apes, Plasmodium schwetzi morphologically appears to be the closest relation to P.ovale. As of 2013 this had not been confirmed by DNA studies.
The original species has been shown to be two morphologically identical forms - Plasmodium ovale curtisi and Plasmodium ovale wallikeri - which can be differentiated only by genetic means.[12] Both species have been identified in Ghana, Myanmar, Nigeria, São Tomé, Sierra Leone and Uganda. The separation of the lineages is estimated to have occurred between 1.0 and 3.5 million years ago in hominid hosts. A second analysis suggests that these species separated 4.5 million years ago (95% confidence interval 0.5 – 7.7 Mya).[15]
These species appear to be more closely related to Plasmodium malariae than to Plasmodium vivax.[15]
The two species appear to differ in their biology with P. ovale wallikeri having a shorter latency period than P. ovale ovale.[16]
Life Cycle
Liver Stage
The P. ovale sporozoite enters a hepatocyte and begins its exoerythrocytic schizogony stage. This is characterized by multiple rounds of nuclear division without cellular segmentation. After a certain number of nuclear divisions, the parasite cell will segment and merozoites are formed.
There are situations where some of the sporozoites do not immediately start to grow and divide after entering the hepatocyte, but remain in a dormant, hypnozoite stage for weeks or months. The duration of latency is variable from one hypnozoite to another and the factors that will eventually trigger growth are not known; this explains how a single infection can be responsible for a series of waves of parasitaemia or "relapses".[17]
Erythrocytic Cycle
While similar to P. vivax, P. ovale is able to infect individuals who are negative for the Duffy blood group, which is the case for many residents of sub Saharan Africa. This explains the greater prevalence of P. ovale (rather than P. vivax) in most of Africa.
[18]
Vectors
- Anopheles albimanus
- Anopheles atroparvus
- Anopheles dirus
- Anopheles farauti
- Anopheles freeborni
- Anopheles gambiae
- Anopheles maculatus
- Anopheles quadrimaculatus
- Anopheles stephensi
- Anopheles subpictus
Other hosts
Chimpanzees and Saimiri monkeys can be infected with this parasite.
See also
List of parasites (human)
References
- ↑ Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, Dolecek C, Hien TT, do Rosário VE, Arez AP, Pinto J, Michon P, Escalante AA, Nosten F, Burke M, Lee R, Blaze M, Otto TD, Barnwell JW, Pain A, Williams J, White NJ, Day NP, Snounou G, Lockhart PJ, Chiodini PL, Imwong M, Polley SD (2010). "Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally". J Infect Dis 201 (10): 1544–50. doi:10.1086/652240. PMID 20380562.
- ↑ Stephens JWW (1922) A new malaria parasite of man. Ann Trop Med Parasitol 16: 383–388. doi: 10.1098/rspb.1914.0024
- ↑ Cornu M, Combe A, Couprie B et al. (1986). "[Epidemiological aspects of malaria in 2 villages of the Manyemen forest region (Cameroon, southwest province)]". Med Trop (Mars) (in French) 46 (2): 131–40. PMID 3523108.
- ↑ Faye FB, Konaté L, Rogier C, Trape JF (1998). "Plasmodium ovale in a highly malaria endemic area of Senegal". Trans. R. Soc. Trop. Med. Hyg. 92 (5): 522–5. doi:10.1016/S0035-9203(98)90900-2. PMID 9861368.
- ↑ Baird JK, Hoffman SL (November 2004). "Primaquine therapy for malaria". Clin. Infect. Dis. 39 (9): 1336–45. doi:10.1086/424663. PMID 15494911.
- ↑ Fuehrer HP, Starzengruber P, Swoboda P et al. (2010). "Indigenous Plasmodium ovale malaria in Bangladesh". Am. J. Trop. Med. Hyg. 83 (1): 75–78. doi:10.4269/ajtmh.2010.09-0796. PMC 2912579. PMID 20595481.
- ↑ Incardona S, Chy S, Chiv L et al. (June 2005). "Large sequence heterogeneity of the small subunit ribosomal RNA gene of Plasmodium ovale in Cambodia". Am. J. Trop. Med. Hyg. 72 (6): 719–24. PMID 15964956.
- ↑ Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN (April 1993). "Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections". Mol. Biochem. Parasitol. 58 (2): 283–92. doi:10.1016/0166-6851(93)90050-8. PMID 8479452.
- ↑ Cadigan FC, Desowitz RS (1969). "Two cases of Plasmodium ovale malaria from central Thailand". Trans. R. Soc. Trop. Med. Hyg. 63 (5): 681–2. doi:10.1016/0035-9203(69)90194-1. PMID 5824291.
- ↑ Gleason NN, Fisher GU, Blumhardt R, Roth AE, Gaffney GW (1970). "Plasmodium ovale malaria acquired in Viet-Nam". Bull. World Health Organ. 42 (3): 399–403. PMC 2427544. PMID 4392940.
- ↑ Lysenko AJ, Beljaev AE (1969). "An analysis of the geographical distribution of Plasmodium ovale". Bull. World Health Organ. 40 (3): 383–94. PMC 2554635. PMID 5306622.
- ↑ 12.0 12.1 Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, et al. (2010) Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis 201: 1544–155
- ↑ Fuehrera H-P, Noedl H (2014). "Recent advances in detection of Plasmodium ovale: Implications of separation into the two species Plasmodium ovale wallikeri and Plasmodium ovale curtisi.". J Clin Microbiol 52 (2): 387–391. doi:10.1128/JCM.02760-13.
- ↑ Radloff PD, Philipps J, Hutchinson D, Kremsner PG (1996). "Atovaquone plus proguanil is an effective treatment for Plasmodium ovale and P. malariae malaria". Trans R Soc Trop Med Hyg 90 (6): 682. doi:10.1016/S0035-9203(96)90435-6. PMID 9015517.
- ↑ 15.0 15.1 Putaporntip C, Hughes AL, Jongwutiwes S (2013) Low level of sequence diversity at merozoite surface protein-1 locus of Plasmodium ovale curtisi and P. ovale wallikeri from Thai isolates. PLoS One 8(3):e58962. doi: 10.1371/journal.pone.0058962
- ↑ Nolder D, Oguike MC, Maxwell-Scott H, Niyazi HA, Smith V, Chiodini PL, Sutherland CJ (2013) An observational study of malaria in British travellers: Plasmodium ovale wallikeri and Plasmodium ovale curtisi differ significantly in the duration of latency. BMJ Open 3(5). pii: e002711. doi: 10.1136/bmjopen-2013-002711
- ↑ "Malaria eModule - Exo-Erythrocytic Stages".
- ↑ "Biology: Malaria (CDC malaria)".
External links
- Malaria - TDR: For research on diseases of poverty
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||