Photon upconversion
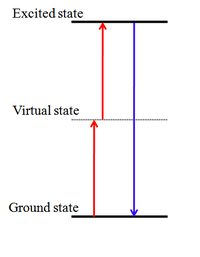

Photon upconversion (UC) is a process in which the sequential absorption of two or more photons leads to the emission of light at shorter wavelength than the excitation wavelength. It is an anti-Stokes type emission. An example is the conversion of infrared light to visible light.[1][2][3] Materials by which upconversion can take place often contain ions of d-block and f-block elements. Examples of these ions are Ti2+, Ni2+, Mo3+, Re4+, and Os4+.
Three basic mechanisms are energy transfer upconversion, excited-state absorption (ESA) and photon avalanche (PA). Upconversion should be distinguished from two-photon absorption and second-harmonic generation. An early proposal (a solid-state IR quantum counter) was made by N. Bloembergen in 1959[4] The process was first observed by F. Auzel in 1966[5][6]
Thermal upconversion mechanism has also been proposed. This mechanism is based on the absorption of photons with low energies in the upconverter, which heats up and re-emits photons with higher energies. To make this process possible, the density of optical states of the upconverter has to be carefully engineered to provide frequency- and angularly-selective emission characteristics. For example, a planar thermal upconverting platform can have a front surface that absorbs low-energy photons incident within a narrow angular range, and a back surface that efficiently emits only high-energy photons. These surface properties can be realized through designs of photonic crystal, and theories and experiments have been demonstrated on thermophotovoltaics and radiation cooling.[7][8] Under best criterion, energy conversion efficiency from solar radiation to electricity by introducing up-converter can go up to 73% using AM1.5D spectrum and 76% considering sun as a blackbody source at 6000K for a single junction cell.[9]
Upconversion nanoparticles
Lanthanide-doped nanoparticles emerged in the late 1990s due to the prevalent work on nanotechnology, marking a turning point in the landscape of modern lanthanide research. Although the optical transitions in lanthanide-doped nanoparticles essentially resemble those in bulk materials, the nanostructure amenable to surface modifications provides new opportunities for research. Particularly, these nanoparticles are promising alternatives to molecular fluorophores for bioapplications. Their unique optical properties, such as large Stokes shift and nonblinking, have enabled them to rival conventional luminescent probes in challenging tasks including single-molecule tracking and deep tissue imaging. Despite the promising aspects of these nanomaterials, one urgent task that confronts materials chemists lies in the synthesis of nanoparticles with tunable emissions, which are essential for applications in multiplexed imaging and sensing.[10]
Applications and examples
At present, there is great interest in luminescent materials for efficient frequency conversion from infrared to visible radiation, mainly because a visible source pumped by a near infrared laser is useful for high-capacity data storage optical devices. This process can be obtained by upconversion mechanisms, where several infrared photons can be absorbed by the material doped with rare earth ions (RE) in order to populate more energetic levels. Therefore, both the fluorescence lifetime and the stimulated emission cross-section of the RE excited level should be maximized, whereas the nonradiative decay mechanisms should be minimized. Oxyfluoride glass ceramics are ambivalent materials. Despite the fact that they are mainly oxide glasses, they can exhibit optical properties of fluoride single crystals when they are doped with rare earth ions. They are often called nanocomposite materials. Their weird character is obtained by a classical melting and quenching preparation in air followed by an adapted thermal treatment during which fluoride phases are crystallized. The size, size distribution, and volume concentration of fluoride crystallites are crucial for photonic applications. For example, to be a promising optical functional material, the size of the crystallites should be smaller than at least half of the wavelength of the light used while the size distribution should be narrow and the crystallites should possess a homogeneous spatial distribution. In this way, according to the scattering theory developed by Rayleigh, complete transparency of a light transmitting material can be attained. A refractive index difference between the amorphous and crystalline phases of less than 0.1 is also required. However, according to Beall and Pinckney, based on Hopper’s model, crystal sizes of 30 nm and differences in refractive index of 0.3 may be acceptable, provided that the crystal spacing is not larger than six times the average crystal size. Transparent Glass Ceramic (TGC) can also be obtained with even larger crystal sizes if optical isotropy is achieved within the glass ceramic. Consequently, the selection of the oxide glass composition and the fluoride phase composition is the key factor in obtaining the desired glass ceramic materials. The Er3+ ions are specially interesting due to their emission at 1.5 μm and the green upconversion obtained under near infrared excitation. In order to improve these emissions, the sensitization of this nanocomposite with Yb3+ ions may be a good choice because of the efficient energy transfer process from Yb3+ to Er3+ ions.[11][12]
References
- ↑ Haase, M.; Schäfer, H. (2011). "Upconverting Nanoparticles". Angewandte Chemie International Edition 50: 5808–5829. doi:10.1002/anie.201005159.
- ↑ Auzel, François (2004). "Upconversion and Anti-Stokes Processes with f and d Ions in Solids". Chem. Rev. 104 (1): 139–174. doi:10.1021/cr020357g.
- ↑ Design of Luminescent Inorganic Materials: New Photophysical Processes Studied by Optical Spectroscopy Daniel R. Gamelin and Hans U. Güdel Acc. Chem. Res., 2000, 33 (4), pp 235–242 doi:10.1021/ar990102y
- ↑ Bloembergen, N (1959). "Solid State Infrared Quantum Counters". Phys. Rev. Lett 2: 84. Bibcode:1959PhRvL...2...84B. doi:10.1103/PhysRevLett.2.84.
- ↑ F. Auzel, C. R. Acad" Sci 1966, 262, 1016
- ↑ F. Auzel, C. R. Acad Sci 1966, 263, 819
- ↑ Raman, A. P. et al. (2014). Nature 515: 540–544. doi:10.1038/nature13883. Missing or empty
|title=(help) - ↑ Lenert, A. et al. (2014). Nature Nanotechnology 9: 126–130. doi:10.1038/nature13883. Missing or empty
|title=(help) - ↑ S.V. Boriskina, G. Chen, 2014, 314, 71–78, doi:10.1016/j.optcom.2013.10.042
- ↑ Wang, F.; Liu, X. "Multicolor Tuning of Lanthanide-Doped Nanoparticles by Single Wavelength Excitation". Accounts of Chemical Research 2014. doi:10.1021/ar5000067.
- ↑ Imanieh, Mohammad H.; Martín, Inocencio R.; Eftekhari Yekta, Bijan; Marghussian, Vahak K.; Shakhesi, Saeed; Pinckney, L. (December 2012). "Improved Cooperative Emission in Ytterbium-Doped Oxyfluoride Glass-Ceramics Containing CaF Nanocrystals". Journal of the American Ceramic Society 95 (12): 3827–3833. doi:10.1111/jace.12012.
- ↑ Imanieh, M. H.; Martín, I. R.; Gonzalez-Platas, J.; Eftekhari Yekta, B.; Marghussian, V. K.; Shakhesi, S. (2014). "Behavior of Yb3+ and Er3+ during Heat Treatment in Oxyfluoride Glass Ceramics". Journal of Nanomaterials 2014: 1–10. doi:10.1155/2014/171045.