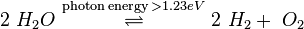Photocatalytic water splitting
Photocatalytic water splitting is an artificial photosynthesis process with photocatalysis in a photoelectrochemical cell used for the dissociation of water into its constituent parts, hydrogen (H
2) and oxygen (O
2), using either artificial or natural light. Theoretically, only solar energy (photons), water, and a catalyst are needed.
Hydrogen fuel production has gained increased attention as oil and other nonrenewable fuels become increasingly depleted and expensive. Methods such as photocatalytic water splitting are being investigated to produce hydrogen fuel, which burns cleanly and can be used in a hydrogen fuel cell. Water splitting holds particular interest since it utilizes water, an inexpensive renewable resource. Photocatalytic water splitting has the simplicity of using a powder in solution and sunlight to produce H
2 and O
2 from water and can provide a clean, renewable energy, without producing greenhouse gases or having many adverse effects on the atmosphere.
Concepts
When H
2O is split into O
2 and H
2, the stoichiometric ratio of its products is 2:1:
The process of water-splitting is a highly endothermic process (ΔH > 0). Water splitting occurs naturally in photosynthesis when photon energy is absorbed and converted into the chemical energy through a complex biological pathway. However, production of hydrogen from water requires large amounts of input energy, making it incompatible with existing energy generation. For this reason, most commercially produced hydrogen gas is produced from natural gas.
There are several strict requirements for a photocatalyst to be useful for water splitting. The minimum potential difference (voltage) needed to split water is 1.23V at 0 pH.[1] Since the minimum band gap for successful water splitting at pH=0 is 1.23 eV, corresponding to light of 1008 nm, the electrochemical requirements can theoretically reach down into infrared light, albeit with negligible catalytic activity. These values are true only for a completely reversible reaction at standard temperature and pressure (1 bar and 25 °C).
Theoretically, infrared light has enough energy to split water into hydrogen and oxygen; however, this reaction is kinetically very slow because the wavelength is greater than 380 nm. The potential must be less than 3.0V to make efficient use of the energy present across the full spectrum of sunlight. Water splitting can transfer charges, but not be able to avoid corrosion for long term stability. Defects within crystalline photocatalysts can act as recombination sites, ultimately lowering efficiency.
Materials used in photocatalytic water splitting fulfill the band requirements outlined previously and typically have dopants and/or co-catalysts added to optimize their performance. A sample semiconductor with the proper band structure is titanium dioxide (TiO
2). However, due to the relatively positive conduction band of TiO
2, there is little driving force for H
2 production, so TiO
2 is typically used with a co-catalyst such as platinum (Pt) to increase the rate of H
2 production. It is routine to add co-catalysts to spur H
2 evolution in most photocatalysts due to the conduction band placement. Most semiconductors with suitable band structures to split water absorb mostly UV light; in order to absorb visible light, it is necessary to narrow the band gap. Since the conduction band is fairly close to the reference potential for H
2 formation, it is preferable to alter the valence band to move it closer to the potential for
O
2 formation, since there is a greater natural overpotential.[2]
Photocatalysts can suffer from catalyst decay and recombination under operating conditions. Catalyst decay becomes a problem when using a sulfide-based photocatalyst such as cadmium sulfide (CdS), as the sulfide in the catalyst is oxidized to elemental sulfur at the same potentials used to split water. Thus, sulfide-based photocatalysts are not viable without sacrificial reagents such as sodium sulfide to replenish any sulfur lost, which effectively changes the main reaction to one of hydrogen evolution as opposed to water splitting. Recombination of the electron-hole pairs needed for photocatalysis can occur with any catalyst and is dependent on the defects and surface area of the catalyst; thus, a high degree of crystallinity is required to avoid recombination at the defects.[2]
The conversion of solar energy to hydrogen by means of photocatalysis is one of the most interesting ways to achieve clean and renewable energy systems. However if this process is assisted by photocatalysts suspended directly in water instead of using a photovoltaic and electrolytic system the reaction is in just one step, and can therefore be more efficient.[3][4]
Method of evaluation
Photocatalysts must conform to several key principles in order to be considered effective at water splitting. A key principle is that H
2 and O
2 evolution should occur in a stoichiometric 2:1 ratio; significant deviation could be due to a flaw in the experimental setup and/or a side reaction, both of which do not indicate a reliable photocatalyst for water splitting. The prime measure of photocatalyst effectiveness is quantum yield (QY), which is:
- QY (%) = (Photochemical reaction rate) / (Photon absorption rate) × 100%[2]
This quantity is a reliable determination of how effective a photocatalyst is; however, it can be misleading due to varying experimental conditions. To assist in comparison, the rate of gas evolution can also be used; this method is more problematic on its own because it is not normalized, but it can be useful for a rough comparison and is consistently reported in the literature. Overall, the best photocatalyst has a high quantum yield and gives a high rate of gas evolution.
The other important factor for a photocatalyst is the range of light absorbed; though UV-based photocatalysts will perform better per photon than visible light-based photocatalysts due to the higher photon energy, far more visible light reaches the Earth's surface than UV light. Thus, a less efficient photocatalyst that absorbs visible light may ultimately be more useful than a more efficient photocatalyst absorbing solely light with smaller wavelengths.
Photocatalyst systems
NaTaO
3:La
NaTaO
3:La yields the highest water splitting rate of photocatalysts without using sacrificial reagents.[2] This UV-based photocatalyst was shown to be highly effective with water splitting rates of 9.7 mmol/h and a quantum yield of 56%. The nanostep structure of the material promotes water splitting as edges functioned as H
2 production sites and the grooves functioned as O
2 production sites. Addition of NiO particles as cocatalysts assisted in H
2 production; this step was done by using an impregnation method with an aqueous solution of Ni(NO
3)
2•6H
2O and evaporating the solution in the presence of the photocatalyst. NaTaO
3 has a conduction band higher than that of NiO, so photogenerated electrons are more easily transferred to the conduction band of NiO for H
2 evolution.[5]
K
3Ta
3B
2O
12
K
3Ta
3B
2O
12, another catalyst activated by solely UV light and above, does not have the performance or quantum yield of NaTaO
3:La. However, it does have the ability to split water without the assistance of cocatalysts and gives a quantum yield of 6.5% along with a water splitting rate of 1.21 mmol/h. This ability is due to the pillared structure of the photocatalyst, which involves TaO
6 pillars connected by BO
3 triangle units. Loading with NiO did not assist the photocatalyst due to the highly active H
2 evolution sites.[6]
(Ga
.82Zn
.18)(N
.82O
.18)
(Ga
.82Zn
.18)(N
.82O
.18) has the highest quantum yield in visible light for visible light-based photocatalysts that do not utilize sacrificial reagents as of October 2008.[2] The photocatalyst gives a quantum yield of 5.9% along with a water splitting rate of 0.4 mmol/h. Tuning the catalyst was done by increasing calcination temperatures for the final step in synthesizing the catalyst. Temperatures up to 600 °C helped to reduce the number of defects, though temperatures above 700 °C destroyed the local structure around zinc atoms and was thus undesirable. The treatment ultimately reduced the amount of surface Zn and O defects, which normally function as recombination sites, thus limiting photocatalytic activity. The catalyst was then loaded with Rh
2-yCr
yO
3 at a rate of 2.5 wt % Rh and 2 wt% Cr to yield the best performance.[7]
Pt/TiO
2
TiO
2 is a very efficient photocatalyst, as it yields both a high quantum number and a high rate of H
2 gas evolution. For example, Pt/TiO2 (anatase phase) is a catalyst used in water splitting. These photocatalysts combine with a thin NaOH aqueous layer to make a solution that can split water into H
2 and O
2. TiO
2 absorbs only ultraviolet light due to its large band gap(>3.0ev), but outperforms most visible light photocatalysts because it does not photocorrode as easily. Most ceramic materials have large band gaps and thus have stronger covalent bonds than other semiconductors with lower band gaps.
Cobalt based systems
Photocatalysts based on cobalt have been reported.[8] Members are tris(bipyridine) cobalt(II), compounds of cobalt ligated to certain cyclic polyamines, and certain cobaloximes.
In 2014 researchers announced an approach that connected a chromophore to part of a larger organic ring that surrounded a cobalt atom. The process is less efficient than using a platinum catalyst, cobalt is less expensive, potentially reducing total costs. The process uses one of two supramolecular assemblies based on Co(II)-templated coordination of Ru(bpy)32+ (bpy = 2,2′-bipyridyl) analogues as photosensitizers and electron donors to a cobaloxime macrocycle. The Co(II) centres of both assemblies are high spin, in contrast to most previously described cobaloximes. Transient absorption optical spectroscopies include that charge recombination occurs through multiple ligand states present within the photosensitizer modules.[9][10]
Bismuth
Bismuth based systems have been demonstrated to have an efficiency of 5% with the advantage of a very simple and cheap catalyst.[11]
References
- ↑ J. Head, J. Turner, “ANALYSIS OF THE WATER-SPLITTING CAPABILITIES OF GALLIUM INDIUM PHOSPHIDE NITRIDE (GaInPN)” U.S. Department of Energy Journal of Undergraduate Research, January 2001, 26-31. Web link.
- ↑ 2.0 2.1 2.2 2.3 2.4 A. Kudo, Y. Miseki, “Heterogeneous photocatalyst materials for water splitting” Chem. Soc. Rev., 38, 253-278 (2009). Web link.
- ↑ del Valle, F.; Álvarez Galván, M. Consuelo; Del Valle, F.; Villoria De La Mano, José A.; Fierro, José L. G. et al. (Jun 2009). "Water Splitting on Semiconductor Catalysts under Visible-Light Irradiation". Chemsuschem (CHEMSUSCHEM) 2 (6): 471–485. doi:10.1002/cssc.200900018. PMID 19536754.
- ↑ del Valle, F.; Del Valle, F.; Villoria De La Mano, J.A.; Álvarez-Galván, M.C.; Fierro, J.L.G. et al. (2009). "Photocatalytic water splitting under visible Light: concept and materials requirements". Advances in Chemical Engineering 36: 111–143. doi:10.1016/S0065-2377(09)00404-9.
- ↑ H. Kato, K. Asakura, A. Kudo, “Highly Efficient Water Splitting into H and O over Lanthanum-Doped NaTaO Photocatalysts with High Crystallinity and Surface Nanostructure” J. Am. Chem. Soc., 125, 3082 (2003).
- ↑ T. Kurihara, H. Okutomi, Y. Miseki, H. Kato, A. Kudo, “Highly Efficient Water Splitting over K
3Ta
3B
2O
12Photocatalyst without Loading Cocatalyst” Chem. Lett., 35, 274 (2006). - ↑ K. Maeda, K. Teramura, K. Domen,
“Effect of post-calcination on photocatalytic activity of (Ga
1-xZn
x)(N
1-xO
x) solid solution for overall water splitting under visible light” J. Catal., 254, 198 (2008). - ↑ Artero, V.; Chavarot-Kerlidou, M.; Fontecave, M. (2011). "Splitting Water with Cobalt". Angewandte Chemie International Edition 50 (32): 7238. doi:10.1002/anie.201007987.
- ↑ "A less-expensive way to duplicate the complicated steps of photosynthesis in making fuel". KurzweilAI. doi:10.1039/C3CP54420F. Retrieved 2014-01-23.
- ↑ Mukherjee, A.; Kokhan, O.; Huang, J.; Niklas, J.; Chen, L. X.; Tiede, D. M.; Mulfort, K. L. (2013). "Detection of a charge-separated catalyst precursor state in a linked photosensitizer-catalyst assembly". Physical Chemistry Chemical Physics (Royal Society of Chemistry) 15 (48): 21070–21076. doi:10.1039/C3CP54420F. PMID 24220293.
- ↑ Abdi, Fatwa F; Lihao Han; Arno H. M. Smets; Miro Zeman; Bernard Dam; Roel van de Krol (29 July 2013). "Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode". Nature Communications. Bibcode:2013NatCo...4E2195A. doi:10.1038/ncomms3195. Retrieved 5 August 2013.