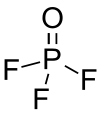
Phosphoryl fluoride
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Phosphoryl trifluoride Phosphorus trifluoride oxide | |||
| Other names
Phosphorus oxyfluoride Phosphoric trifluoride | |||
| Identifiers | |||
| 13478-20-1 | |||
| ChemSpider | 75351 | ||
| EC number | 236-776-4 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 83516 | ||
| |||
| Properties | |||
| POF3 | |||
| Molar mass | 103.9684 g/mol | ||
| Appearance | Clear, colourless gas | ||
| Boiling point | −39.7 °C (−39.5 °F; 233.5 K) | ||
| Reacts | |||
| Solubility | Reacts with alcohol and acid soluble in ether and hydrocarbons | ||
| Structure | |||
| Molecular shape | tetrahedral | ||
| Dipole moment | D | ||
| Hazards | |||
| MSDS | ICSC 0190 | ||
| Main hazards | Poison, corrosive, can form HF on contact with H2O | ||
| EU Index | 015-009-00-5 | ||
| EU classification | | ||
| R-phrases | R14, R34, R36/37/38[1] | ||
| S-phrases | (S1/2), S7/9, S26, S36/37/39, S45 | ||
| NFPA 704 | |||
| Related compounds | |||
| Related compounds |
Thiophosphoryl fluoride Phosphoryl chloride Phosphorus oxybromide Phosphorus trifluoride Phosphorus pentafluoride | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| Infobox references | |||
Phosphoryl fluoride (commonly called phosphorus oxyfluoride) is a compound with the chemical formula POF3. It is a toxic gas.
Reactions
Phosphoryl fluoride combines with dimethylamine to produce dimethylaminophosphoryldifluoride (CH3)2NPOF2 and difluorophosphate and hexafluorophosphate ions.[2]
References
- ↑ http://www.chemicalbook.com/ProductChemicalPropertiesCB3329830_EN.htm
- ↑ Cavell, R. G. (1968). "Chemistry of phosphorus fluorides. Part III. The reaction of thiophosphoryl-fluoride with dimethylamine and some properties of the dimethylaminothio- phosphoryl fluorides". Canadian Journal of Chemistry 46 (4): 613. doi:10.1139/v68-100. Retrieved 2 Feb 2012.
| Wikimedia Commons has media related to Phosphoryl fluoride. |