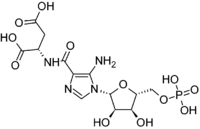
Phosphoribosylaminoimidazolesuccinocarboxamide
 | |
| Names | |
|---|---|
| Systematic IUPAC name
2-[(5-Amino-1-{3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl}-1H-imidazol-4-yl)formamido]butanedioic acid | |
| Other names
SAICAR; 2-[(5-Amino-1-{3,4-dihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl}imidazol-4-yl)formamido]butanedioic acid; N-{[5-Amino-1-(5-O-phosphono-β-D-ribofuranosyl)-1H-imidazol-4-yl]carbonyl}-L-aspartic acid | |
| Identifiers | |
| 3DMet | B04963 |
| 3031-95-6 | |
| ChEBI | CHEBI:18319 |
| ChemSpider | 141175 |
| |
| Jmol-3D images | Image |
| KEGG | C04823 |
| MeSH | SAICAR |
| PubChem | 160666 |
| |
| Properties | |
| Molecular formula |
C13H19N4O12P |
| Molar mass | 454.28 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Phosphoribosylaminoimidazolesuccinocarboxamide (SAICAR) is an intermediate in the formation of purines. The conversion of ATP, L-aspartate, and 5-aminoimidazole-4-carboxyribonucleotide (CAIR) to 5-aminoimidazole-4-(N-succinylcarboxamide) ribonucleotide, ADP, and phosphate by phosphoribosylaminoimidazolesuccinocarboxamide synthetase (SAICAR synthetase) represents the eighth step of de novo purine nucleotide biosynthesis.[1]
References
- ↑ Scott W. Nelson, Daniel J. Binkowski, Richard B. Honzatko, and Herbert J. Fromm (2005). "Mechanism of Action of Escherichia coli Phosphoribosylaminoimidazolesuccinocarboxamide Synthetase". Biochemistry 44 (2): 766–774. doi:10.1021/bi048191w. PMID 15641804.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||