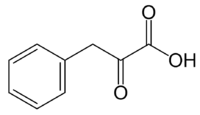
Phenylpyruvic acid
 | |
| Names | |
|---|---|
| IUPAC name
2-Oxo-3-phenylpropanoic acid | |
| Other names
Phenylpyruvate; 3-Phenylpyruvic acid; Keto-phenylpyruvate; beta-Phenylpyruvic acid | |
| Identifiers | |
| 156-06-9 | |
| ChEBI | CHEBI:30851 |
| ChemSpider | 972 |
| |
| Jmol-3D images | Image |
| PubChem | 997 |
| |
| Properties | |
| Molecular formula |
C9H8O3 |
| Molar mass | 164.16 g·mol−1 |
| Melting point | 155 °C (311 °F; 428 K) (decomposes) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Phenylpyruvic acid is a pyruvic acid derivative.
Synthesis
Phenylpyruvic acid is synthesized from benzyl chloride by double carbonylation (82% yield).[1][2]
See also
References
- ↑ Wolfram, Joachim. "Preparation of α-keto-carboxylic acids from acyl halides". Google Patents US4481368 & US4481369. Ethyl Corporation.
- ↑ Werner Bertleff, Michael Roeper, & Xavier Sava (2007). "Carbonylation". Ullmann's Encyclopedia of Industrial Chemistry: pg.19. doi:10.1002/14356007.a05_217.pub2.