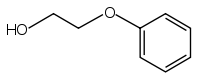
Phenoxyethanol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Phenoxyethanol | |
| Other names
Phenoxyethanol Ethylene glycol monophenyl ether Phenoxytolarosol Dowanol EP / EPH Emery 6705 Rose ether 1-Hydroxy-2-phenoxyethane β-hydroxyethyl phenyl ether Phenyl cellosolve | |
| Identifiers | |
| 122-99-6 | |
| ChEBI | CHEBI:64275 |
| ChEMBL | ChEMBL1229846 |
| ChemSpider | 13848467 |
| |
| Jmol-3D images | Image |
| PubChem | 31236 |
| |
| UNII | HIE492ZZ3T |
| Properties | |
| Molecular formula |
C8H10O2 |
| Molar mass | 138.16 g·mol−1 |
| Appearance | Colorless oily liquid |
| Density | 1.102 g/cm3 |
| Melting point | 11 °C (52 °F; 284 K) |
| Boiling point | 247 °C (477 °F; 520 K) |
| Hazards | |
| NFPA 704 | |
| Flash point | 113 °C (235 °F) (closed cup) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Phenoxyethanol is chemical preservative, a glycol ether often used in dermatological products such as skin creams and sunscreen. It is a colorless oily liquid. It is a bactericide (usually used in conjunction with quaternary ammonium compounds), often used in place of sodium azide in biological buffers because phenoxyethanol is less toxic and non-reactive with copper and lead. It is used in many applications such as cosmetics, vaccines and pharmaceuticals as a preservative.
Application
It is also used as a fixative for perfumes, an insect repellent, a topical antiseptic, a solvent for cellulose acetate, some dyes, inks, and resins, in preservatives, pharmaceuticals, and in organic synthesis. It is moderately soluble in water. It is used as an anesthetic in the aquaculture of some fish.[1][2]
It is also listed as an ingredient for many United States vaccines by the Center for Disease Control.[3] In Japan and the EU, its usage level in cosmetic products is restricted to concentrations of up to 1%.[4][5]
Efficacy
The activity of the preservative phenoxyethanol was effective in inactivating challenge doses of gram-negative and gram-positive microorganisms, as well as a yeast (Candida albicans).[6]
Safety
Phenoxyethanol is an alternative to standard, potentially harmful formaldehyde-releasing preservatives.[7]
In 2005–06, methyldibromoglutaronitrile/ phenoxyethanol was the ninth-most-prevalent allergen in patch tests (5.8%).[8]
The Food and Drug Administration has warned that the chemical is toxic to infants via ingestion, and "can depress the central nervous system and may cause vomiting and diarrhea." Combined with Chlorphenesin, these two chemicals can cause respiratory depression in infants.[9] Since these chemicals are often present in cosmetics and lotions applied to the hands and are easily ingested, caution should be exercised.
German research in 1999, concluded that it had neurotoxin potential, but in a concentration-dependent manner.[10]
The EPA (Environmental Protection Agency) data sheets show chromosomal changes and genetic mutation effects in testing as well as testicular atrophy and interference with reproductivity in mice for other Glycol Ethers, although Phenoxyethanol is not mentioned in the abstract.[11]
References
- ↑ Tsantilas, H.; Galatos, A.D.; Athanassopoulou, F.; Prassinos, N.N.; Kousoulaki, K. (2006). "Efficacy of 2-phenoxyethanol as an anaesthetic for two size classes of white sea bream, Diplodus sargus L., and sharp snout sea bream, Diplodus puntazzo C". Aquaculture 253 (1–4): 64–70. doi:10.1016/j.aquaculture.2005.07.034. INIST:17608707.
- ↑ Mylonas C., Cardilanetti G., Sigelaki I., Polzonetti-Magni A. (2005). "Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of european sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures". Aquaculture 246 (1–4): 467–81. doi:10.1016/j.aquaculture.2005.02.046. INIST:16750867.
- ↑ CDC excipient table
- ↑ Tokunaga H, Takeuchi O, Ko R, Uchino T, Ando M (2003). "市販化粧水中のフェノキシエタノールおよびパラベン類の分析法に関する研究" [Studies for analyzing phenoxyethanol and parabens in commercial lotions] (PDF). Kokuritsu Iyakuhin Shokuhin Eisei Kenkyūjo Hōkoku (in Japanese) (121): 25–9. PMID 14740401.
- ↑ http://ec.europa.eu/consumers/cosmetics/cosing/index.cfm?fuseaction=search.details&id=36522&back=1[]
- ↑ Lowe I, Southern J (1994). "The antimicrobial activity of phenoxyethanol in vaccines". Lett Appl Microbiol 18 (2): 115–6. doi:10.1111/j.1472-765X.1994.tb00820.x. PMID 7764595.
- ↑ Wineski LE, English AW (1989). "Phenoxyethanol as a nontoxic preservative in the dissection laboratory". Acta Anat (Basel) 136 (2): 155–8. doi:10.1159/000146816. PMID 2816264.
- ↑ ZZug KA, Warshaw EM, Fowler JF et al. (2009). "Patch-test results of the North American Contact Dermatitis Group 2005-2006". Dermatitis 20 (3): 149–60. PMID 19470301.
- ↑ "FDA Warns Consumers Against Using Mommy's Bliss Nipple Cream".
- ↑ Schmuck G, Steffens W, Bomhard E (July 2000). "2-Phenoxyethanol: a neurotoxicant?". Archives of Toxicology 74 (4-5): 281–7. doi:10.1007/s002040000110. PMID 10959804.
- ↑ Hardin BD (June 1983). "Reproductive toxicity of the glycol ethers". Toxicology 27 (2): 91–102. doi:10.1016/0300-483X(83)90014-8. PMID 6351353.

