Phenanthrenoid
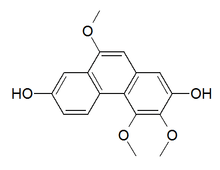
Phenanthreoids are chemical compounds formed with a phenanthrene backbone. Those compound are naturally occurring in plants, although they can also be synthetized.[1]
Phenanthrols
Phenanthrols are any of five isomeric phenols derived from phenanthrene (1-Phenanthrol, 2-Phenanthrol, 3-Phenanthrol, 4-Phenanthrol, 9-Phenanthrol). These molecules can be biomarkers of smoking and/or PAH worker exposure.[2]
Chemistry
Under UV irradiation, stilbene and its derivatives undergo intramolecular cyclization to form dihydrophenanthrenes.
Natural occurrences
Phenanthrenes have been reported from higher plants, mainly in the Orchidaceae family. A few phenanthrenes have been found in the Hepaticae class and Dioscoreaceae, Combretaceae and Betulaceae families.[3]
The rhizome of Dioscorea communis contains phenanthrenes (7-hydroxy-2,3,4,8-tetramethoxyphenanthrene, 2,3,4-trimethoxy-7,8-methylenedioxyphenanthrene, 3-hydroxy-2,4,-dimethoxy-7,8-methylenedioxyphenanthrene, 2-hydroxy-3,5,7-trimethoxyphenanthrene and 2-hydroxy-3,5,7-trimethoxy-9,10-dihydrophenanthrene).[4]
The dimeric phenanthrenoid 8,8'-bidehydrojuncusol and the monomeric dehydrojuncusol can be isolated from Juncus acutus.[5]
Perakensol is a phenanthrenoid that can be isolated from Alseodaphne perakensis.[6]
In orchids
Phenanthrenes have been reported in species of Dendrobium, Bulbophyllum, Eria, Maxillaria, Bletilla, Coelogyne, Cymbidium, Ephemerantha and Epidendrum.[3]
3,4,8-Trimethoxyphenanthrene-2,5-diol is one of the 17 phenanthrenes found in the extract of the stems of the orchid Dendrobium nobile.[7][8]
From the stems of the orchid Flickingeria fimbriata, three phenanthrenes can be isolated. The structures are 2,5-dihydroxy-4,9,10-trimethoxyphenanthrene, 2,5-dihydroxy-4-methoxyphenanthrene and 2,5,9-trihydroxy-4-methoxy-9,10-dihydrophenanthrene. These molecules are named plicatol A, B and C.[9]
Nudol is a phenanthrene of the orchids Eulophia nuda, Eria carinata and Eria stricta.[10] 9,10-Dihydro-2,5-dimethoxyphenanthrene-1,7-diol is a phenanthrene from Eulophia nuda. This compound shows cytotoxic activity against human cancer cells.[11]
2,7-Dihydroxy-3,6-dimethoxyphenanthrene is a phenanthrene from Dehaasia longipedicellata.[12]
Bulbophyllum gymnopus produces the phenanthrenediol gymnopusin.[13]
Bulbophyllum reptans contains gymnopusin, confusarin (2,7-dihydroxy-3,4,8-trimethoxyphenanthrene), 2,7-dihydroxy-3,4,6-trimethoxyphenanthrene and its 9,10-dihydro derivative, flavanthrinin (2,7-dihydroxy-4-methoxyphenanthrene) and its 9,10-dihydro derivative (coelonin), cirrhopetalanthrin (2,2′,7,7′-tetrahydroxy-4,4′-dimethoxy-1,1′-biphenanthryl), its 9,9′,10,10′-tetrahydro derivative (flavanthrin) and the dimeric phenanthrenes reptanthrin and isoreptanthrin.[14]
Bulbophyllum vaginatum contains the two phenanthrenes 4,9-dimethoxyphenanthrene-2,5-diol and 4,6-dimethoxyphenanthrene-2,3,7-triol, and the two dihydrophenanthrenes 4-methoxy-9,10-dihydrophenanthrene-2,3,7-triol and 4,6-dimethoxy-9,10-dihydrophenanthrene-2,3,7-triol.[15]
Coelogyne cristata contains coeloginanthridin (3,5,7-trihydroxy-1,2-dimethoxy-9,10-dihydrophenanthrene), a 9,10-dihydrophenanthrene derivative, and coeloginanthrin (3,5,7-trihydroxy-1,2-dimethoxyphenanthrene), the corresponding phenanthrene analogue, coelogin and coeloginin.[16]
Orchinol and loroglossol have a phytoalexin effect and reduce the growth of Cattleya aurantiaca seedlings.[17]
The phenanthrenes 2,5-dihydroxy-3,4-dimethoxyphenanthrene, 9,10-dihydro-2,5-dihydroxy-3,4-dimethoxyphenanthrene, 2,7-dihydroxy-3,4-dimethoxyphenanthrene (nudol), 9,10-dihydro-2,7-dihydroxy-3,4-dimethoxyphenanthrene, 2,5-dihydroxy-3,4,9-trimethoxyphenanthrene and 2,7-dihydroxy-3,4,9-trimethoxyphenanthrene can be isolated from Maxillaria densa.[18]
Cirrhopetalanthrin is a dimeric phenanthrene derivative from Cirrhopetalum maculosum.[19]
Glycosides
Five phenanthrene glycosides, denneanoside A, B, C, D and E and one 9,10-dihydrophenanthrene glycoside, denneanoside F, can be isolated from the stem of Dendrobium denneanum.[20]
Metabolism
Cis-3,4-dihydrophenanthrene-3,4-diol dehydrogenase is an enzyme that uses (+)-cis-3,4-dihydrophenanthrene-3,4-diol and NAD+ to produce phenanthrene-3,4-diol, NADH and H+. This enzyme participates in naphthalene and anthracene degradation.
See also
- (+)-Cavicularin, a cyclic bibenzyl-dihydrophenanthrene derivative[21]
References
- ↑ Evans, David A.; Cain, Paul A.; Wong, Rayman Y. (1977). "A general approach to the synthesis of phenanthrenoid compounds. An alternative to oxidative phenolic coupling". J. Am. Chem. Soc 99 (21): 7083–7085. doi:10.1021/ja00463a063.
- ↑ Serdar, B; Waidyanatha, S; Zheng, Y; Rappaport, SM (2003). "Simultaneous determination of urinary 1- and 2-naphthols, 3- and 9-phenanthrols, and 1-pyrenol in coke oven workers". Biomarkers 8 (2): 93–109. doi:10.1080/1354750021000046570. PMID 12775495.
- ↑ 3.0 3.1 Natural phenanthrenes and their biological activity. Adriána Kovács, Andrea Vasas, Judit Hohmann, Phytochemistry, March 2008, Volume 69, Issue 5, Pages 1084–1110, doi:10.1016/j.phytochem.2007.12.005
- ↑ Phenanthrenes and a dihydrophenanthrene from Tamus communis and their cytotoxic activity. Adriána Kovácsa, Peter Forgob, István Zupkóc, Borbála Réthyc, György Falkayc, Pál Szabód and Judit Hohmanna, Phytochemistry, Volume 68, Issue 5, March 2007, Pages 687–691, doi:10.1016/j.phytochem.2006.10.028
- ↑ FA1, Behery; Naeem, ZE; Maatooq, GT; Amer, MM; Ahmed, AF (2013). "A novel antioxidant phenanthrenoid dimer from Juncus acutus L.". Nat Prod Res. 27 (2): 155–163. doi:10.1080/14786419.2012.662759. PMID 22360833.
- ↑ Mahmud, Zurinah; Khan, Mohammad N.; Lajis, Nordin H.; Toia, Robert F. (1992). "Perakensol: A Phenanthrenoid Isolated from Alseodaphne perakensis". J. Nat. Prod. 55 (4): 533–535. doi:10.1021/np50082a027.
- ↑ Hwang JS, Lee SA, Hong SS, Han XH, Lee C, Kang SJ, Lee D, Kim Y, Hong JT, Lee MK, Hwang BY,."Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorg Med Chem Lett. 2010 Jun 15;20(12):3785-7, doi:10.1016/j.bmcl.2010.04.054
- ↑ Yang H., Sang H.S., Young C.K."Antifibrotic phenanthrenes of Dendrobium nobile stems" Journal of Natural Products 2007 70:12 (1925-1929)
- ↑ Phenanthrenes from Dendrobium plicatile. Chie Honda and Masae Yamaki, Phytochemistry, April 2000, Volume 53, Issue 8, Pages 987–990, doi:10.1016/S0031-9422(99)00497-5
- ↑ Nudol, a phenanthrene of the orchids Eulophia nuda, Eria carinata and Eria stricta. S Bhandari, Phytochemistry, January 1985, volume 24, issue 4, pages 801-804, doi:10.1016/S0031-9422(00)84898-0
- ↑ Cytotoxic activity of 9,10-dihydro-2,5-dimethoxyphenanthrene-1,7-diol from Eulophia nuda against human cancer cells. Varsha Shriram, Vinay Kumar, P B Kavi Kishor, Sharad B Suryawanshi, Ankur K Upadhyay and Manoj K Bhat, Journal of ethnopharmacology, March 2010, volume 128, issue 1, pages 251-253, doi:10.1016/j.jep.2009.12.031
- ↑ 2,7-Dihydroxy-3,6-dimethoxyphenanthrene from Dehaasia longipedicellata. Mat Ropi Mukhtar, Mohd Azlan Nafiah, Khalijah Awang, A. Hamid A. Hadi and Seik Weng Ng, ACTA CRYSTALLOGR E-STRUCT REP, January 2008, volume 64, issue 6, doi:10.1107/S1600536808014451/bt2712Isup2.hkl
- ↑ Structure and synthesis of gymnopusin, a novel phenanthrenediol from the orchid Bulbophyllum gymnopus. Andrew B. Hughes and Melvyn V. Sargent, J. Chem. Soc., 1989, Perkin Trans. 1, pages 1787-1791, doi:10.1039/P19890001787
- ↑ Dimeric phenanthrenes from the orchid Bulbophyllum reptans. P.L Majumder, S Pal and S Majumder, Phytochemistry, Volume 50, Issue 5, 10 March 1999, Pages 891–897, doi:10.1016/S0031-9422(98)00609-8
- ↑ Leong Y-W, KAng C-C; Harrison, LJ; Powell, AD (1997). "Phenanthrenes, dihydrophenanthrenes and bibenzyls from the orchid Bulbophyllum vaginatum". Phytochemistry 44 (1): 157–165. INIST:2557322.
- ↑ "Phenanthrene derivatives from the orchid Coelogyne cristata.". Phytochemistry 58 (4): 581–6. Oct 2001. doi:10.1016/s0031-9422(01)00287-4. PMID 11576602.
- ↑ Effects of Orchinol, Loroglossol, Dehydroorchinol, Batatasin III, and 3,4'- Dihydroxy-5-Methoxydihydrostilbene on Orchid Seedlings. Katherine A. Hills, Albert Stoessl, Allison P. Oliva and Joseph Arditti, Botanical Gazette, September 1984, Vol. 145, No. 3, pages 298-301 (link)
- ↑ New Phenanthrene Derivatives from Maxillaria densa. Samuel Estrada, Rubén A. Toscano and Rachel Mata, J. Nat. Prod., 1999, volume 62, issue 8, pages 1175–1178, doi:10.1021/np990061e
- ↑ Cirrhopetalanthrin, a dimeric phenanthrene derivative from the orchid Cirrhopetalum maculosum. P.L. Majumder, Anjali Pal, Mukta Joardar, Phytochemistry, Volume 29, Issue 1, 1990, Pages 271–274, doi:10.1016/0031-9422(90)89048-E
- ↑ New phenanthrene glycosides from Dendrobium denneanum and their cytotoxic activity. Fu Li, Hong-Mei Pan, Xin Liu, Bin Chen, Ya-Xiong Tang, Xing-Jun Xi and Ming-Kui Wang, Phytochemistry Letters, Volume 6, Issue 4, November 2013, Pages 640–644, doi:10.1016/j.phytol.2013.08.003
- ↑ M. Toyota, T. Yoshida, Y. Kan, S. Takaoka, Y. Asakawa (1996). "(+)-Cavicularin: A Novel Optically Active Cyclic Bibenzyl-Dihydrophenanthrene Derivative from the Liverwort Cavicularia densa Steph". Tetrahedron Letters 37 (27): 4745–4748. doi:10.1016/0040-4039(96)00956-2.
External links
-
 The dictionary definition of phenanthrol at Wiktionary
The dictionary definition of phenanthrol at Wiktionary
| ||||||
| ||||||||||||||||||||||||||||||||