Perrhenic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Perrhenic(VII) acid | |
| Other names
Hydrated rhenium(VII) oxide | |
| Identifiers | |
| 13768-11-1 | |
| ChemSpider | 21106462 |
| |
| Jmol-3D images | Image |
| RTECS number | TT4550000 |
| |
| Properties | |
| H4O9Re2 (solid) HReO4 (gas) | |
| Molar mass | 251.2055 g/mol |
| Appearance | Pale yellow solid |
| Density | ? |
| Melting point | °C (? K) |
| Boiling point | sublimes |
| Soluble | |
| Acidity (pKa) | -1.25[1] |
| Structure | |
| octahedral-tetrahedral (solid) tetrahedral (gas) | |
| Hazards | |
| Main hazards | Corrosive |
| R-phrases | R34 |
| S-phrases | S26, S36/37, S39, S45 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
| Related compounds |
Re2O7, Mn2O7 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
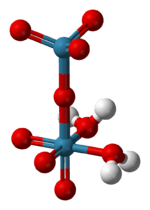
Perrhenic acid is the chemical compound with the formula Re2O7(OH2)2. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam.[2] When a solution of Re2O7 is kept for a period of months, it breaks down and crystals of HReO4.H2O are formed, which contain tetrahedral ReO4−[3] For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
Properties
The structure of solid perrhenic acid is [O3Re-O-ReO3(H2O)2].[4] This species is a rare example of a metal oxide coordinated to water - most often metal-oxo-aquo species are unstable with respect to the corresponding hydroxides:
- M(O)(H2O) → M(OH)2
Gaseous perrhenic acid is tetrahedral, as suggested by its formula HReO4.
Reactions
Perrhenic acid or the related anhydrous oxide Re2O7 converts to dirhenium heptasulfide upon treatment with hydrogen sulfide:
- Re2O7+ 7 H2S → Re2S7 + 7 H2O
The heptasulfide, which has a complex structure,[5] catalyses the hydrogenation of double bonds and is useful because it tolerates sulfur compounds, which poison noble metal catalysts. Re2S7 also catalyses the reduction of nitric oxide to N2O.
Perrhenic acid in the presence of HCl undergoes reduction in the presence of thioethers and tertiary phosphines to give Re(V) complexes with the formula ReOCl3L2.[6]
Perrhenic acid combined with platinum on a support gives rise to a useful hydrogenation and hydrocracking catalyst for the petroleum industry.[7] For example, silica impregnated with a solution of perrhenic acid is reduced with hydrogen at 500 °C. This catalyst is used in the dehydrogenation of alcohols and also promotes the decomposition of ammonia.
Catalysis
Perrhenic acid is a precursor to a variety of homogeneous catalysts, some of which are promising in niche applications that can justify the high cost of rhenium. In combination with tertiary arsines, perrhenic acid gives a catalyst for the epoxidation of alkenes with hydrogen peroxide.[8] Perrhenic acid catalyses the dehydration of oximes to nitriles.[9]
Other uses
Perrhenic acid is also used in the manufacture of x-ray targets.[10][11]
See also
References
- ↑ http://www.iupac.org/publications/pac/1998/pdf/7002x0355.pdf
- ↑ Glemser, O.; Müller, A.; Schwarzkopf, H. (1964). "Gasförmige Hydroxide. IX. Über ein Gasförmiges Hydroxid des Rheniums". Zeitschrift für anorganische und allgemeine Chemie 334: 21–26. doi:10.1002/zaac.19643340105..
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ Beyer, H.; Glemser, O.; Krebs, B. “Dirhenium Dihydratoheptoxide Re2O7(OH2)2 - New Type of Water Bonding in an Aquoxide” Angewandte Chemie, International Edition English 1968, Volume 7, Pages 295 - 296. doi:10.1002/anie.196802951.
- ↑ Schwarz, D. E.; Frenkel, A. I.; Nuzzo, R. G.; Rauchfuss, T. B.; Vairavamurthy, A. (2004). "Electrosynthesis of ReS4. XAS Analysis of ReS2, Re2S7, and ReS4". Chemistry of Materials 16: 151–158. doi:10.1021/cm034467v.
- ↑ Parshall, G. W.; Shive, L. W.; Cotton, F. A. (1997). "Phosphine Complexes of Rhenium". Inorganic Syntheses 17: 110–112. doi:10.1002/9780470132487.ch31.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ van Vliet, M. C. A.; Arends, I. W. C. E.; Sheldon, R. A. (1999). "Rhenium Catalysed Epoxidations with Hydrogen Peroxide: Tertiary Arsines as Effective Cocatalysts". J. Chem. Soc., Perkin Trans. 1 (3): 377–80. doi:10.1039/a907975k.
- ↑ Ishihara, K.; Furuya, Y.; Yamamoto, H. (2002). "Rhenium(VII) Oxo Complexes as Extremely Active Catalysts in the Dehydration of Primary Amides and Aldoximes to Nitriles". Angewandte Chemie, International Edition 41 (16): 2983–2986. doi:10.1002/1521-3773(20020816)41:16<2983::AID-ANIE2983>3.0.CO;2-X.
- ↑ http://www.gehealthcare.com/usen/service/time_material_support/docs/Radplus2100.pdf
- ↑ X-ray#Sources
| ||||||||||||||||||||||||||||||||||||||||

