Perfluorotributylamine
 | |
| Names | |
|---|---|
| IUPAC name
1,1,2,2,3,3,4,4,4-nonafluoro-N,N-bis(1,1,2,2,3,3,4,4,4-nonafluorobutyl)butan-1-amine | |
| Other names
Fluorinert | |
| Identifiers | |
| 311-89-7 | |
| ChEBI | CHEBI:38854 |
| ChemSpider | 13836523 |
| |
| Jmol-3D images | Image |
| PubChem | 9397 |
| |
| Properties | |
| Molecular formula |
C12F27N |
| Molar mass | 671.09 g·mol−1 |
| Density | 1.884 g/mL |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 178 °C (352 °F; 451 K) |
| Insoluble | |
| Solubility in methanol and isopropyl alcohol | Insoluble |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Perfluorotributylamine (PFTBA) is a liquid whose molecular structure consists of three butyl moieties connected to an amine center, in which the hydrogen atoms have all been replaced with fluorine. The compound is produced by the electronic industry, along with other perfluoroalkyl amines.[1] It is used as an ingredient in Fluosol and in some Fluorinert coolant liquids.[2]
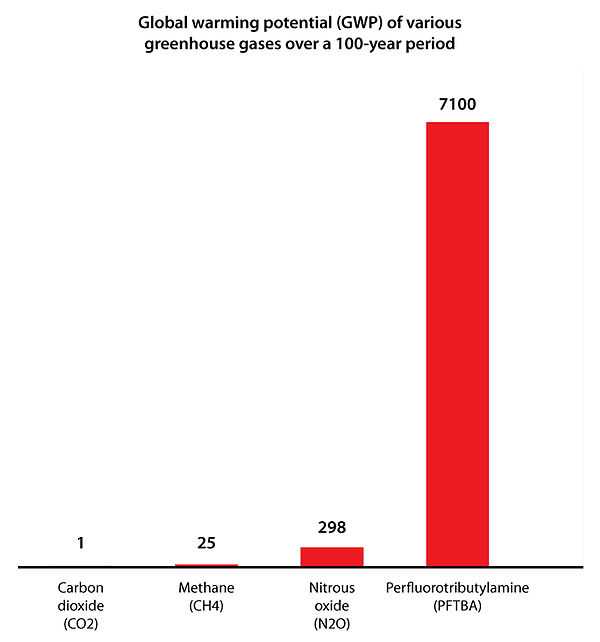
In 2013, this liquid was shown by researchers at the University of Toronto to be a greenhouse gas, with warming properties more than 7,000 times that of carbon dioxide over a 100 year period,[3][4] and that, as such, it is the most potent greenhouse gas ever discovered.[5] Its concentration in the atmosphere is approximately 0.18 parts per trillion. The researchers also reported that the gas can persist in the atmosphere for up to 500 years.
See also
References
- ↑ Upton, John (10 December 2013). "Meet perfluorotributylamine, the world’s worst greenhouse gas". Grist. Retrieved 11 December 2013.
- ↑ Garrelts, J. C. (1990). "Fluosol: An oxygen-delivery fluid for use in percutaneous transluminal coronary angioplasty". DICP : the annals of pharmacotherapy 24 (11): 1105–1112. PMID 2275237.
- ↑ Hong, A. C.; Young, C. J.; Hurley, M. D.; Wallington, T. J.; Mabury, S. A. (2013). "Perfluorotributylamine: A novel long-lived greenhouse gas". Geophysical Research Letters: n/a. doi:10.1002/2013GL058010.
- ↑ Goldenberg, Suzanne (10 December 2013). "Newly discovered greenhouse gas '7,000 times more powerful than CO2'". The Guardian. Retrieved 11 December 2013.
- ↑ Goldenberg, Suzanne (11 December 2013). "Newly Discovered Greenhouse Gas "7,000 Times More Powerful than CO2"". Mother Jones. Retrieved 12 December 2013.