Paraformaldehyde
 | |
| Names | |
|---|---|
| IUPAC name
Polyoxymethylene | |
| Identifiers | |
| 30525-89-4 | |
| ChemSpider | |
| PubChem | 24898648 |
| Properties | |
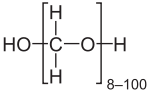
| OH(CH2O)nH (n = 8 - 100) | |
| Appearance | white crystalline solid |
| Density | 1.42 g·cm−3 (25 °C) |
| Melting point | 120 °C (248 °F; 393 K) |
| low | |
| Hazards | |
| MSDS | Oxford MSDS |
| EU classification | Toxic (T); Corrosive (C) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Paraformaldehyde (PFA) is the smallest polyoxymethylene, the polymerization product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition. Paraformaldehyde is a poly-acetal.
Synthesis
Paraformaldehyde forms slowly in aqueous formaldehyde solutions as a white precipitate, especially if stored in the cold. Formalin actually contains very little monomeric formaldehyde; most of it forms short chains of polyformaldehyde. A small amount of methanol is often added as a stabilizer to limit the extent of polymerization.
Reactions
Paraformaldehyde can be depolymerized to formaldehyde gas by dry heating[1] and to form a formaldehyde solution by water in the presence of a base or heat. The very pure formaldehyde solutions obtained in this way are used as a fixative for microscopy and histology.
The resulting formaldehyde gas from dry heating paraformaldehyde is flammable.
Uses
Once paraformaldehyde is depolymerized, the resulting formaldehyde may be used as a fumigant, disinfectant, fungicide, and fixative. Longer chain-length (high molecular weight) polyoxymethylenes are used as a thermoplastic and are known as polyoxymethylene plastic (POM, Delrin). It was used in the past in the discredited Sargenti method of root canal treatment.[2]
Paraformaldehyde is not a fixative; it must be depolymerized to formaldehyde in solution. In cell culture, a typical formaldehyde fixing procedure would involve using a 4% formaldehyde solution in phosphate buffered saline (PBS) on ice for 10 minutes.
Toxicity
As a formaldehyde releasing agent, paraformaldehyde is a suspected carcinogen. Its acute oral median lethal dose in rats is 592 mg/kg.
See also
- Polyoxymethylene plastic
- 1,3,5-Trioxane, the cyclic trimer of formaldehyde
- Polymer
References
- ↑ Yates, J (1973). "Adsorption and decomposition of formaldehyde on tungsten (100) and (111) crystal planes". Journal of Catalysis 30 (2): 260. doi:10.1016/0021-9517(73)90073-0.
- ↑ http://www.dentalwatch.org/questionable/sargenti/overview.html