PEDOT:PSS
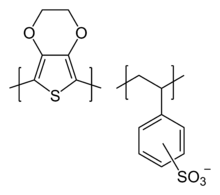
PEDOT:PSS or poly(3,4-ethylenedioxythiophene) polystyrene sulfonate is a polymer mixture of two ionomers. One component in this mixture is made up of sodium polystyrene sulfonate which is a sulfonated polystyrene. Part of the sulfonyl groups are deprotonated and carry a negative charge. The other component poly(3,4-ethylenedioxythiophene) or PEDOT is a conjugated polymer and carries positive charges and is based on polythiophene. Together the charged macromolecules form a macromolecular salt.[1]
It is used as a transparent, conductive polymer with high ductility in different applications. For example, AGFA coats 200 million photographic films per year with a thin, extensively-stretched layer of virtually transparent and colorless PEDOT:PSS as an antistatic agent to prevent electrostatic discharges during production and normal film use, independent of humidity conditions.
If organic compounds, including high boiling solvents like methylpyrrolidone, dimethyl sulfoxide, sorbitol, ionic liquids and surfactants, are added conductivity increases by many orders of magnitude.[2][3][4][5]This makes it also suitable as a transparent electrode, for example in touchscreens, organic light-emitting diodes and electronic paper to replace the traditionally used indium tin oxide (ITO). Owing to the high conductivity (up to 1000 S/cm), it can be used as a cathode material in capacitors replacing manganese dioxide or liquid electrolytes. It is also used in organic electrochemical transistors.
The conductivity of PEDOT:PSS can also be significantly improved by a post-treatment with various compounds, such as ethylene glycol, dimethyl sulfoxide (DMSO), salts, zwitterions, cosolvents, acids, geminal diols and amphiphilic fluoro- compounds.[6] This conductivity is comparable to that of ITO, the popular transparent electrode material, and it can triple that of ITO after a network of carbon nanotubes and silver nanowires is embedded into PEDOT:PSS.[7]
This compound is generally applied as a dispersion of gelled particles in water. A conductive layer on glass is obtained by spreading a layer of the dispersion on the surface usually by spin coating and driving out the water by heat. Special PEDOT:PSS inks and formulations were developed for different coating and printing processes. Water-based PEDOT:PSS inks are mainly used in slot die coating, flexography, rotogravure and inkjet printing. If a high viscous paste and slow drying is required like in screen-printing processes PEDOT:PSS can also be supplied in high boiling solvents like propanediol. Dry PEDOT:PSS pellets can be produced with a freeze drying method which are redispersable in water and different solvents, for example ethanol to increase drying speed during printing. Finally, to overcome degradation to ultraviolet light and high temperature or humidity conditions PEDOT:PSS UV-stabilizers are available.
See also
References
- ↑ Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J. R. (2000). "Poly(3,4-ethylenedioxythiophene) and Its Derivatives: Past, Present, and Future". Advanced Materials 12 (7): 481. doi:10.1002/(SICI)1521-4095(200004)12:7<481::AID-ADMA481>3.0.CO;2-C.
- ↑ Kim, J. Y.; Jung, J. H.; Lee, D. E.; Joo, J. (2002). "Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents". Synthetic Metals 126 (2–3): 311. doi:10.1016/S0379-6779(01)00576-8.
- ↑ Ouyang, J.; Xu, Q.; Chu, C. W.; Yang, Y.; Li, G.; Shinar, J. (2004). "On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment". Polymer 45 (25): 8443. doi:10.1016/j.polymer.2004.10.001.
- ↑ Döbbelin, M.; Marcilla, R.; Salsamendi, M.; Pozo-Gonzalo, C.; Carrasco, P. M.; Pomposo, J. A.; Mecerreyes, D. (2007). "Influence of Ionic Liquids on the Electrical Conductivity and Morphology of PEDOT:PSS Films". Chemistry of Materials 19 (9): 2147. doi:10.1021/cm070398z.
- ↑ Xia, Y; Ouyang, J (2010). "Significant conductivity enhancement of conductive poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) films through a treatment with organic carboxylic acids and inorganic acids". ACS Applied Materials & Interfaces 2 (2): 474–83. doi:10.1021/am900708x. PMID 20356194.
- ↑ Ouyang, J.; Chu, C. -W.; Chen, F. -C.; Xu, Q.; Yang, Y. (2005). "High-Conductivity Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) Film and Its Application in Polymer Optoelectronic Devices". Advanced Functional Materials 15 (2): 203. doi:10.1002/adfm.200400016.
- ↑ Stapleton, A. J.; Yambem, S. D.; Johns, A. H.; Afre, R. A.; Ellis, A. V.; Shapter, J. G.; Andersson, G. G.; Quinton, J. S.; Burn, P. L.; Meredith, P.; Lewis, D. A. (2015). "Planar silver nanowire, carbon nanotube and PEDOT:PSS nanocomposite transparent electrodes". Science and Technology of Advanced Materials 16 (2): 025002. doi:10.1088/1468-6996/16/2/025002.