PDK3
Pyruvate dehydrogenase lipoamide kinase isozyme 3, mitochondrial is an enzyme that in humans is encoded by the PDK3 gene.[1][2] It codes for an isozyme of pyruvate dehydrogenase kinase.The pyruvate dehydrogenase (PDH) complex is a nuclear-encoded mitochondrial multienzyme complex that catalyzes the overall conversion of pyruvate to acetyl-CoA and CO(2). It provides the primary link between glycolysis and the tricarboxylic acid (TCA) cycle, and thus is one of the major enzymes responsible for the regulation of glucose metabolism. The enzymatic activity of PDH is regulated by a phosphorylation/dephosphorylation cycle, and phosphorylation results in inactivation of PDH. The protein encoded by this gene is one of the four pyruvate dehydrogenase kinases that inhibits the PDH complex by phosphorylation of the E1 alpha subunit. This gene is predominantly expressed in the heart and skeletal muscles. Alternatively spliced transcript variants encoding different isoforms have been found for this gene.[2]
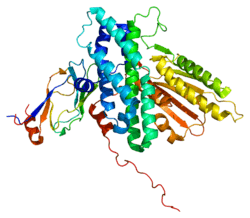
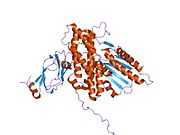
Structure
The structure of the PDK3/L2 complex has been elucidated, and there are several key features. When the L2 domain binds to PDK3, it induces a “cross-tail” conformation in PDK3, thereby stimulating activity. There are three crucial residues, Leu-140, Glu-170, and Glu-179, in the C-terminal domain that are crucial for this interaction.[3] Structural studies have indicated that L2 binding stimulates activity by disrupting the closed conformation, or ATP lid, to remove product inhibition.[4] The PDK3 subunits are in one of two conformations; one subunit exists as an “open” subunit, while the other subunit is “closed”. The open subunit is the configuration most crucial to the putative substrate-binding cleft, as it is where the target peptide can access the active center. The closed subunit blocks this target peptide because of a neighboring unwound alpha helix. Additionally, the ATP-binding loop in one PDK3 subunit adopts an open conformation, implying that the nucleotide loading into the active site is mediated by the inactive "pre-insertion" binding mode. This asymmetric complex represents a physiological state in which binding of a single L2-domain activates one of the PDHK subunits while inactivating another.[5] Thus, the L2-domains likely act not only as the structural anchors but also modulate the catalytic cycle of PDK3.
Function
The Pyruvate Dehydrogenase (PDH) complex must be tightly regulated due to its central role in general metabolism. Within the complex, there are three serine residues on the E1 component that are sites for phosphorylation; this phosphorylation inactivates the complex. In humans, there have been four isozymes of Pyruvate Dehydrogenase Kinase that have been shown to phosphorylate these three sites: PDK1, PDK2, PDK3, and PDK4.[6] The PDK3 protein is primarily found in the kidney, brain, and testis.[7]
Regulation
As the primary regulators of a crucial step in the central metabolic pathway, the pyruvate dehydrogenase family is tightly regulated itself by a myriad of factors. PDK3, in conjunction with PDK2 and PDK4, are primary targets of Peroxisome proliferator-activated receptor delta or beta, with PDK3 having five elements that respond to these receptors.[8]
References
- ↑ Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM (Jan 1996). "Diversity of the pyruvate dehydrogenase kinase gene family in humans". J Biol Chem 270 (48): 28989–94. doi:10.1074/jbc.270.48.28989. PMID 7499431.
- ↑ 2.0 2.1 "Entrez Gene: PDK3 pyruvate dehydrogenase kinase, isozyme 3".
- ↑ Tso SC, Kato M, Chuang JL, Chuang DT (Sep 2006). "Structural determinants for cross-talk between pyruvate dehydrogenase kinase 3 and lipoyl domain 2 of the human pyruvate dehydrogenase complex". The Journal of Biological Chemistry 281 (37): 27197–204. doi:10.1074/jbc.M604339200. PMID 16849321.
- ↑ Kato M, Chuang JL, Tso SC, Wynn RM, Chuang DT (May 2005). "Crystal structure of pyruvate dehydrogenase kinase 3 bound to lipoyl domain 2 of human pyruvate dehydrogenase complex". The EMBO Journal 24 (10): 1763–74. doi:10.1038/sj.emboj.7600663. PMID 15861126.
- ↑ Devedjiev Y, Steussy CN, Vassylyev DG (Jul 2007). "Crystal structure of an asymmetric complex of pyruvate dehydrogenase kinase 3 with lipoyl domain 2 and its biological implications". Journal of Molecular Biology 370 (3): 407–16. doi:10.1016/j.jmb.2007.04.083. PMID 17532006.
- ↑ Kolobova E, Tuganova A, Boulatnikov I, Popov KM (Aug 2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". The Biochemical Journal 358 (Pt 1): 69–77. PMID 11485553.
- ↑ Sugden MC, Holness MJ (Jul 2002). "Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia". Current Drug Targets. Immune, Endocrine and Metabolic Disorders 2 (2): 151–65. PMID 12476789.
- ↑ Degenhardt T, Saramäki A, Malinen M, Rieck M, Väisänen S, Huotari A et al. (Sep 2007). "Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta". Journal of Molecular Biology 372 (2): 341–55. doi:10.1016/j.jmb.2007.06.091. PMID 17669420.
Further reading
- Sugden MC, Holness MJ (2003). "Therapeutic potential of the mammalian pyruvate dehydrogenase kinases in the prevention of hyperglycaemia". Curr. Drug Targets Immune Endocr. Metabol. Disord. 2 (2): 151–65. doi:10.2174/1568008023340785. PMID 12476789.
- Sugden MC, Holness MJ (2003). "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs". Am. J. Physiol. Endocrinol. Metab. 284 (5): E855–62. doi:10.1152/ajpendo.00526.2002 (inactive 2015-01-01). PMID 12676647.
- Baker JC, Yan X, Peng T, Kasten S, Roche TE (2000). "Marked differences between two isoforms of human pyruvate dehydrogenase kinase". J. Biol. Chem. 275 (21): 15773–81. doi:10.1074/jbc.M909488199. PMID 10748134.
- Kolobova E, Tuganova A, Boulatnikov I, Popov KM (2001). "Regulation of pyruvate dehydrogenase activity through phosphorylation at multiple sites". Biochem. J. 358 (Pt 1): 69–77. doi:10.1042/0264-6021:3580069. PMC 1222033. PMID 11485553.
- Korotchkina LG, Patel MS (2001). "Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase". J. Biol. Chem. 276 (40): 37223–9. doi:10.1074/jbc.M103069200. PMID 11486000.
- Tuganova A, Boulatnikov I, Popov KM (2002). "Interaction between the individual isoenzymes of pyruvate dehydrogenase kinase and the inner lipoyl-bearing domain of transacetylase component of pyruvate dehydrogenase complex". Biochem. J. 366 (Pt 1): 129–36. doi:10.1042/BJ20020301. PMC 1222743. PMID 11978179.
- Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D (2005). "Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting". J. Appl. Physiol. 96 (6): 2082–7. doi:10.1152/japplphysiol.01318.2003. PMID 14966024.
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP et al. (2006). "Genomic Analysis of Mouse Retinal Development". PLoS Biol. 2 (9): E247. doi:10.1371/journal.pbio.0020247. PMC 439783. PMID 15226823.
- Kato M, Chuang JL, Tso SC, Wynn RM, Chuang DT (2005). "Crystal structure of pyruvate dehydrogenase kinase 3 bound to lipoyl domain 2 of human pyruvate dehydrogenase complex". EMBO J. 24 (10): 1763–74. doi:10.1038/sj.emboj.7600663. PMC 1142596. PMID 15861126.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- Tso SC, Kato M, Chuang JL, Chuang DT (2006). "Structural determinants for cross-talk between pyruvate dehydrogenase kinase 3 and lipoyl domain 2 of the human pyruvate dehydrogenase complex". J. Biol. Chem. 281 (37): 27197–204. doi:10.1074/jbc.M604339200. PMID 16849321.
- Devedjiev Y, Steussy CN, Vassylyev DG (2007). "Crystal Structure of an Asymmetric Complex of Pyruvate Dehydrogenase Kinase 3 with Lipoyl Domain 2 and its Biological Implications". J. Mol. Biol. 370 (3): 407–16. doi:10.1016/j.jmb.2007.04.083. PMC 1994203. PMID 17532006.
- Degenhardt T, Saramäki A, Malinen M, Rieck M, Väisänen S, Huotari A et al. (2007). "Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta". J. Mol. Biol. 372 (2): 341–55. doi:10.1016/j.jmb.2007.06.091. PMID 17669420.
- Kato M, Li J, Chuang JL, Chuang DT (2007). "Distinct Structural Mechanisms for Inhibition of Pyruvate Dehydrogenase Kinase Isoforms by AZD7545, Dichloroacetate, and Radicicol". Structure 15 (8): 992–1004. doi:10.1016/j.str.2007.07.001. PMC 2871385. PMID 17683942.
| |||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||