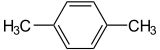
p-Xylene
 | |
 | |
| Names | |
|---|---|
| Other names
p-Xylol 1,4-Dimethylbenzene | |
| Identifiers | |
| 106-42-3 | |
| ChEBI | CHEBI:27417 |
| ChEMBL | ChEMBL31561 |
| ChemSpider | 7521 |
| |
| Jmol-3D images | Image |
| KEGG | C06756 |
| PubChem | 7809 |
| RTECS number | ZE2625000 |
| |
| UNII | 6WAC1O477V |
| Properties | |
| Molecular formula |
C8H10 |
| Molar mass | 106.17 g·mol−1 |
| Appearance | Colorless liquid Colorless crystalline solid |
| Density | 0.861 g/mL |
| Melting point | 13.2 °C (55.8 °F; 286.3 K) |
| Boiling point | 138.35 °C (281.03 °F; 411.50 K) |
| insoluble | |
| Solubility in ethanol | very soluble |
| Solubility in diethyl ether | very soluble |
| Refractive index (nD) |
1.49582 |
| Viscosity | 0.7385 cP at 0 °C 0.6475 cP at 20 °C |
| Dipole moment | 0.07 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Harmful or fatal if swallowed. Vapor harmful. Flammable liquid and vapor. |
| R-phrases | R10 R20 R21 R36 R38 |
| S-phrases | S25 |
| NFPA 704 | |
| Flash point | 25 °C (77 °F; 298 K) |
| Explosive limits | 1.1%-7.0%[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 100 ppm (435 mg/m3)[1] |
| Related compounds | |
| Related aromatic hydrocarbons |
benzene toluene o-xylene m-xylene |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
p-Xylene is an aromatic hydrocarbon based on benzene with two methyl substituents with the chemical formula C8H10 or C6H4(CH3)2. The “p” stands for para, identifying the location of the methyl groups as across from one another. It is an isomer of xylene. Other isomers include o-xylene and m-xylene. The boiling point of p-xylene is 138.35 °C (281 °F) and the melting point is 13.2 °C (56 °F).[2] p-Xylene is used on a large scale for the manufacture of terephthalic acid for polyester. Its polymer is known as parylene.
p-Xylene is produced by catalytic reforming of petroleum naphtha as part of the BTX aromatics (benzene, toluene and the xylene isomers) extracted from the catalytic reformate. The p-xylene is then separated out in a series of distillation, adsorption or crystallization and reaction processes from the m-xylene, o-xylene and ethylbenzene. Its melting point is the highest among this series of isomers, but simple crystallization does not allow easy purification due to the formation of eutectic mixtures. It is also highly flammable.
Nomenclature
p-Xylene is also called 1,4-dimethylbenzene, p-dimethylbenzene; p-xylol; 1,4-xylene; p-methyltoluene; para-sylene; chromar; scintillar; 4-methyltoluene; NSC 72419; 1,4-dimethyl-benzene.[3]
Physical properties
p-Xylene is a colorless, flammable liquid that is insoluble in water with the chemical formula C8H10 or C6H4(CH3)2.[2][4][5] p-Xylene has a boiling point of 138.35 °C (281 °F) and a melting point of 13.2 °C (56 °F). It has a specific gravity of 0.86. p-Xylene is flammable and has a flash point of 27°C (81 °F) or lower. The odor threshold of p-xylene is 0.62 parts per million (ppm).[2]
Exposure to p-Xylene
Inhalation
Inhaling p-xylene can cause dizziness, headache, drowsiness, and nausea. If exposure through inhalation occurs, first aid includes fresh air, rest and possible medical attention. Through the use of ventilation or breathing protection, exposure to p-xylene through inhalation can be prevented.[4]
Skin
Exposure of p-xylene through the skin can cause dry skin and redness. If skin exposure occurs, first aid includes rinsing and then washing the affected area with soap and water as well as removing any contaminated clothing and thoroughly cleaning and drying before reuse. Exposure can be prevented through the use of protective gloves.[4]
Eyes
Exposure of p-xylene to eyes can cause redness and pain. If eyes are exposed, first aid includes rinsing of the eyes with water for several minutes, removal of contact lenses if applicable, and medical attention. Eye exposure can be prevented through the use of safety glasses or safety goggles.[4]
Ingestion
Ingestion of p-xylene can result in a burning sensation, abdominal pain, dizziness, drowsiness, headache, and nausea. If p-xylene is ingested one's mouth should be rinsed and vomiting should NOT be induced. Further medical attention should be sought. Ingestion can be prevented by not eating, drinking, or smoking when working with p-xylene.[4]
Short-term exposure
p-Xylene can cause issues with the central nervous system and if swallowed could cause chemical pneumonitis when breathed into the lungs.[4]
Long-term exposure
Liquid p-xylene exposure to the skin over long periods of time can remove the fat from the skin. The substance may also have effects on the Central Nervous System. Exposure can enhance hearing loss caused by noise exposure. Animal tests suggest that this substance could cause damage to human development and reproductive systems.[4]
Toxicity
Overexposure of p-xylene in humans can cause headache, fatigue, dizziness, listlessness, confusion, irritability, gastrointestinal disturbances including nausea and loss of appetite, flushing of the face, and a feeling of increased body heat. p-Xylene vapor exposure over the recommended exposure limit of 100 parts per million (ppm) can cause irritation to eye, nose, and throat and possible chest tightening and an abnormal gait.[5]
References
- ↑ 1.0 1.1 "NIOSH Pocket Guide to Chemical Hazards #0670". National Institute for Occupational Safety and Health (NIOSH).
- ↑ 2.0 2.1 2.2 "p-Xylene MSDS". ScienceLab.com.
- ↑ "p-Xylene". NIST. Retrieved 21 February 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "para-Xylene". National Institute of Occupational Safety and Health. Retrieved 12 February 2013.
- ↑ 5.0 5.1 "MATERIAL SAFETY DATA SHEET PARA-XYLENE". Amoco. Retrieved 13 February 2013.
