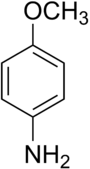
p-Anisidine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
4-Methoxyaniline | |||
| Identifiers | |||
| 104-94-9 | |||
| ChEMBL | ChEMBL295652 | ||
| ChemSpider | 13869414 | ||
| EC-number | 203-254-2 | ||
| |||
| Jmol-3D images | Image | ||
| KEGG | C19326 | ||
| |||
| UN number | 2431 | ||
| Properties[1] | |||
| C7H9NO | |||
| Molar mass | 123.15 g/mol | ||
| Density | 1.071 (57 °C) | ||
| Melting point | 56 to 59 °C (133 to 138 °F; 329 to 332 K) | ||
| Boiling point | 243 °C (469 °F; 516 K) | ||
| soluble | |||
| Solubility | soluble in ethanol, diethyl ether, acetone, benzene | ||
| Vapor pressure | 0.006 mmHg (25°C)[2] | ||
| Refractive index (nD) |
1.5559 | ||
| Hazards | |||
| EU Index | 612-112-00-2 | ||
| Flash point | 122 °C (252 °F; 395 K) | ||
| 515 °C (959 °F; 788 K) | |||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 0.5 mg/m3 [skin][2] | ||
| REL (Recommended) |
TWA 0.5 mg/m3 [skin][2] | ||
| IDLH (Immediate danger) |
50 mg/m3[2] | ||
| Related compounds | |||
| Related compounds |
o-Anisidine m-Anisidine | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
para-Anisidine (p-anisidine), a grey-brown solid, is the most toxic[3] of the three isomers of anisidine and causes blood damage upon oral ingestion, inhalation or skin contact. If heated strongly, it may release very toxic fumes of nitrogen oxides.
p-Anisidine reacts with secondary oxidation products such as aldehydes and ketones in fats and oils to form products that absorb at 350 nm wavelength of light; therefore, it is used as an official method for detecting them by the American Oil Chemists' Society.[4] It is particularly good at detecting unsaturated aldehydes, which are the ones that are most likely to generate unacceptable flavors, making it particularly useful in food quality testing.[5]
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-98. ISBN 0-8493-0462-8..
- ↑ 2.0 2.1 2.2 2.3 "NIOSH Pocket Guide to Chemical Hazards #0035". National Institute for Occupational Safety and Health (NIOSH).
- ↑ http://www.sigmaaldrich.com/catalog/product/aldrich/a88255?lang=de®ion=DE
- ↑ "AOCS Official Method Cd 18-90". American Oil Chemists' Society. Retrieved 26 February 2013.
- ↑ Steele, Robert (2004). Understanding and Measuring the Shelf-Life of Food. Woodhead Publishing in Food Science and Technology Series. Woodhead Publishing. p. 136. ISBN 1855737329.