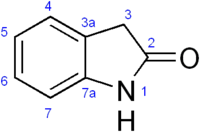
Oxindole
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Oxindole | |
| Systematic IUPAC name
2,3-Dihydro-1H-indol-2-one | |
| Identifiers | |
| 3DMet | B04450 |
| 114692 | |
| 59-48-3 | |
| ChEBI | CHEBI:31697 |
| ChEMBL | ChEMBL40823 |
| ChemSpider | 284794 |
| EC number | 200-429-5 |
| 637057 | |
| |
| Jmol-3D images | Image |
| KEGG | C12312 |
| MeSH | Oxindole |
| PubChem | 321710 |
| RTECS number | NM2080500 |
| |
| Properties | |
| Molecular formula |
C8H7NO |
| Molar mass | 133.15 g·mol−1 |
| Melting point | 128 °C |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Oxindole (2-indolone) is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indoline with a substituted carbonyl at the second position of the 5-member indoline ring.
Oxindole is a tryptophan derivative and in human biology is formed by gut bacteria ("normal flora"). It is normally metabolized and detoxified from the body by the liver. In excess, it can cause sedation, muscle weakness, hypotension, and coma. Patients with hepatic encephalopathy have been recorded to have elevated serum oxindole levels.[1] Recently, oxindoles, as the anticancer agents, have drawn wide attention.[2] An interesting finding is that steroid-oxindole hybrids have shown excellent cytotoxicity against human cancer cell lines and can inhibit cell cycle and induce cell apoptosis.[3][4]
Related aromatic compounds
- Indoline
- Indole
- Indene
- Benzofuran
- Isoindoline
- Carboline
- Isatin
- Methylindole
- Carbazole
- Pyrrole
- Skatole
- Benzene
Precursor
Oxindole is used as a precursor in the synthesis of Amfenac, ..
References
- ↑ Riggio, Oliviero; Mannaioni, Guido; Ridola, Lorenzo; Angeloni, Stefania; Merli, Manuela; Carlà, Vincenzo; Salvatori, Filippo Maria; Moroni, Flavio (2 February 2010). "Peripheral and Splanchnic Indole and Oxindole Levels in Cirrhotic Patients: A Study on the Pathophysiology of Hepatic Encephalopathy". The American Journal of Gastroenterology 105 (6): 1374–1381. doi:10.1038/ajg.2009.738.
- ↑ "Spirooxindoles: Promising scaffolds for anticancer agents". European Journal of Medicinal Chemistry. 27 June 2014. doi:10.1016/j.ejmech.2014.06.056. Retrieved http://www.sciencedirect.com/science/article/pii/S0223523414005856. Check date values in:
|accessdate=(help) - ↑ The Journal of Steroid Biochemistry and Molecular Biology. 6 February 2014. doi:10.1016/j.jsbmb.2014.01.015 http://www.sciencedirect.com/science/article/pii/S0960076014000260. Retrieved http://www.sciencedirect.com/science/article/pii/S0960076014000260. Check date values in:
|accessdate=(help); Missing or empty|title=(help) - ↑ "Discovery of novel steroidal pyran–oxindole hybrids as cytotoxic agents". Steroids. 11 June 2014. doi:10.1016/j.steroids.2014.05.022. Retrieved http://www.sciencedirect.com/science/article/pii/S0039128X14001354. Check date values in:
|accessdate=(help)