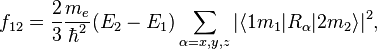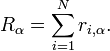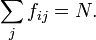Oscillator strength
In spectroscopy, oscillator strength is a dimensionless quantity that expresses the probability of absorption or emission of electromagnetic radiation in transitions between energy levels of an atom or molecule.[1][2][3]
Theory
An atom or a molecule can absorb light and undergo a transition from one quantum state to another.
The oscillator strength  of a transition from a lower state
of a transition from a lower state
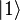 to an upper state
to an upper state 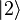 may be defined by
may be defined by
where 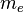 is the mass of an electron and
is the mass of an electron and 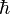 is
the reduced Planck constant. The quantum states
is
the reduced Planck constant. The quantum states 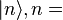 1,2, are assumed to have several
degenerate sub-states, which are labeled by
1,2, are assumed to have several
degenerate sub-states, which are labeled by 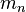 . "Degenerate" means
that they all have the same energy
. "Degenerate" means
that they all have the same energy 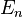 .
The operator
.
The operator 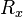 is the sum of the x-coordinates
is the sum of the x-coordinates 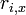 of all
of all 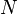 electrons in the system, etc.:
electrons in the system, etc.:
The oscillator strength is the same for each sub-state 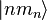 .
.
Thomas–Reiche–Kuhn sum rule
The sum of the oscillator strength from one sub-state 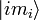 to all
other states
to all
other states 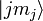 is equal to the number of electrons
is equal to the number of electrons 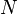 :
:
See also
- Atomic spectral line
- Sum rule in quantum mechanics
References
- ↑ W. Demtröder (2003). Laser Spectroscopy: Basic Concepts and Instrumentation. Springer. p. 31. ISBN 978-3-540-65225-0. Retrieved 26 July 2013.
- ↑ James W. Robinson (1996). Atomic Spectroscopy. MARCEL DEKKER Incorporated. pp. 26–. ISBN 978-0-8247-9742-3. Retrieved 26 July 2013.
- ↑ Hilborn, Robert C. (1982). "Einstein coefficients, cross sections, f values, dipole moments, and all that". American Journal of Physics 50 (11): 982. arXiv:physics/0202029. Bibcode:1982AmJPh..50..982H. doi:10.1119/1.12937. ISSN 0002-9505.
- ↑ Edward Uhler Condon; G. H. Shortley (1951). The Theory of Atomic Spectra. Cambridge University Press. p. 108. ISBN 978-0-521-09209-8. Retrieved 26 July 2013.