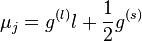Nuclear magnetic moment
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is mainly a magnetic dipole moment; the quadrupole moment does cause some small shifts in the hyperfine structure as well. All nuclei that have a spin also possess a magnetic moment and vice versa, although the connection between the two quantities is not straightforward or easy to calculate.
The nuclear magnetic moment varies from isotope to isotope of an element. Nuclear spin and magnetic moment are both always zero in a ground state (lowest energy) nucleus, if the numbers of protons and of neutrons are both even. In other cases, with odd numbers of either or both protons and neutrons, the nucleus often has spin and magnetic moment.
Shell model
According to the shell model, protons or neutrons tend to form pairs of opposite total angular momentum. Therefore the magnetic moment of a nucleus with even numbers of both protons and neutrons is zero, while that of a nucleus with an odd number of protons and even number of neutrons (or vice versa) will have to be that of the "last", unpaired proton (or neutron). For a nucleus with odd numbers of both protons and neutrons, the total magnetic moment will be some combination of the magnetic moments of both of the "last", unpaired proton and neutron.
Nuclear magnetic moment is only partly predicted by simple versions of the shell model. The magnetic moment is calculated through j, l and s of the "last" nucleon, but nuclei are not in states of well defined l and s. Furthermore, for odd-odd nuclei, one has to consider the two "last" nucleons, as in deuterium. Therefore there are several possible answers for the nuclear magnetic moment, one for each possible combined l and s state, and the real state of the nucleus is a superposition of them. Thus the real (measured) nuclear magnetic moment is between the possible answers, though it may be close to one or the other (as in deuterium).
g-factors
The values of g(l) and g(s) are known as the g-factors of the nucleons.
The measured values of g(l) for the neutron and the proton are according to their electric charge. Thus, in units of nuclear magneton, g(l) = 0 for the neutron and g(l) = 1 for the proton.
The measured values of g(s) for the neutron and the proton are twice their magnetic moment (either the neutron magnetic moment or the proton magnetic moment). In nuclear magneton units, g(s) = -3.8263 for the neutron and g(s) = 5.5858 for the proton.
Calculating the magnetic moment
In the shell model, the magnetic moment of a nucleon of total angular momentum j, orbital angular momentum l and spin s, is given by
Projecting with the total angular momentum j gives
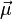 has contributions both from the orbital angular momentum and the spin, with different coefficients g(l) and g(s):
has contributions both from the orbital angular momentum and the spin, with different coefficients g(l) and g(s):
by substituting this back to the formula above and rewriting
For a single nucleon 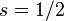 . For
. For 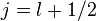 we get
we get
and for 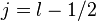
See also
- Gyromagnetic ratio
- Nuclear magneton
- Magnetic moment
- Neutron magnetic moment
- Electron magnetic moment
- Deuterium magnetic moment
- Proton spin crisis
Bibliography
- Nersesov, E.A. (1990). Fundamentals of atomic and nuclear physics. Moscow: Mir Publishers. ISBN 5-06-001249-2.
- Vonsovsky, Sergey V. (1975). Magnetism of Elemetary Particles. Mir Publishers.
External links
-
 Nuclear Structure and Decay Data - IAEA with query on Magnetic Moments
Nuclear Structure and Decay Data - IAEA with query on Magnetic Moments - magneticmoments.info/wp A blog with all recent publications on electromagnetic moments in nuclei
- Table of nuclear magnetic dipole and electric quadrupole moments, N.J. Stone
- RevModPhys Blyn Stoyle
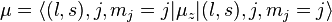
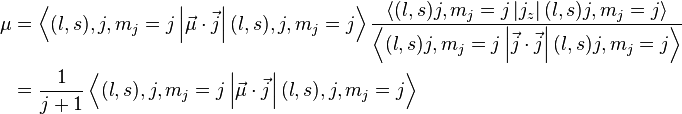
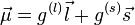
![\begin{align}
\vec{l}\cdot\vec{j} &= \frac{1}{2}\left(\vec{j} \cdot \vec{j} + \vec{l} \cdot \vec{l} - \vec{s} \cdot \vec{s}\right) \\
\vec{s}\cdot\vec{j} &= \frac{1}{2}\left(\vec{j} \cdot \vec{j} - \vec{l} \cdot \vec{l} + \vec{s} \cdot \vec{s}\right) \\
\mu &= \frac{1}{2}\left\langle(l,s),j,m_j=j\left|
g^{(l)}\frac{1}{2}\left(\vec{j} \cdot \vec{j} + \vec{l} \cdot \vec{l} - \vec{s} \cdot \vec{s}\right) +
g^{(s)}\frac{1}{2}\left(\vec{j} \cdot \vec{j} - \vec{l} \cdot \vec{l} + \vec{s} \cdot \vec{s}\right)
\right|(l,s),j,m_j=j\right\rangle \\
&= \frac{1}{j + 1}\left(g^{(l)}\frac{1}{2} \left[j(j + 1) + l(l + 1) - s(s + 1)\right] + g^{(s)}\frac{1}{2} \left[j(j + 1) - l(l + 1) + s(s + 1)\right]\right)
\end{align}](../I/m/4ed0a95c6128ba013bb0f1739badbfb5.png)