Notholaenic acid
 | |
| Names | |
|---|---|
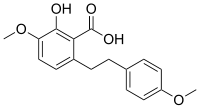
| IUPAC name
2-hydroxy-4-methoxy-6-[2-(4-methoxyphenyl)ethyl]benzoic acid | |
| Identifiers | |
| 72578-97-3 | |
| |
| Jmol-3D images | Image |
| PubChem | 3085829 |
| |
| Properties | |
| Molecular formula |
C17H18O5 |
| Molar mass | 302.32 g·mol−1 |
| Melting point | 149 to 150 °C (300 to 302 °F; 422 to 423 K) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Notholaenic acid is a dihydrostilbenoid found in the farina of some ferns of the genus Notholaena.[1] It has been shown to have anti-HSV-1 activity at high concentrations in vitro.[2] It was artificially synthesized, starting from 3-benzyloxy-5-methoxybenzyl alcohol, in 1985.[3]
References
- ↑ Wollenweber, Eckhard; Favre-Bonvin, Jean (1979). "Novel dihydrostilbene from fronds of Notholaena dealbata and Notholaena limitanea". Phytochemistry 18 (7): 1243–1244. doi:10.1016/0031-9422(79)80153-3.
- ↑ Rinehart, Kenneth L.; Tom G. Holt, Nancy L. Fregeau, Paul A. Keifer, George Robert Wilson, Thomas J. Perun Jr., Ryuichi Sakai, Anthony G. Thompson, Justin G. Stroh, Lois S. Shield, David S. Seigler, Li H. Li, David G. Martin, Cornelis J. P. Grimmelikhuijzen, Gerd Gäde (July–Aug 1990). "Bioactive Compounds from Aquatic and Terrestrial Sources". Journal of Natural Products 53: 771–792. doi:10.1021/np50070a001. Retrieved 29 April 2012. Check date values in:
|date=(help) - ↑ El-Feraly, Farouk S.; Cheatham, Steve F.; McChesney, James D. (1985). "Total Synthesis of Notholaenic Acid". Journal of Natural Products 48 (2): 293–298. doi:10.1021/np50038a015.
| ||||||||||