Noracymethadol
 | |
| Systematic (IUPAC) name | |
|---|---|
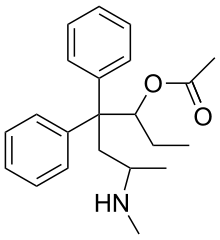
| 6-(Methylamino)-4,4-diphenyl-3-heptanyl acetate | |
| Clinical data | |
| |
| Identifiers | |
|
1477-39-0 5633-25-0 (hydrochloride) 7645-01-4 (gluconate) | |
| None | |
| PubChem | CID 15129 |
| ChemSpider | 14400 |
| Chemical data | |
| Formula | C22H29NO2 |
| 339.471 g/mol | |
|
SMILES
| |
| |
Noracymethadol (INN) is a synthetic opioid analgesic related to methadone that was never marketed.[1] In a clinical trial of postpartum patients it was reported to produce analgesia comparable to that of morphine but with less nausea, dizziness, and drowsiness.[2][3] Other side effects included salivation, ataxia, and respiratory depression that was reversible by naloxone.[2][3] Similarly to many of its analogues, noracymethadol is a Schedule I controlled substance in the United States with an ACSCN of 9633 and 2013 annual manufacturing quota of 12 grammes. [4] and is also controlled internationally under the United Nations Single Convention on Narcotic Drugs of 1961.[5]
Noracymethadol is an acetyl ester of methadol and it can be said with some precison that it is either the heroin or 6-monoacetylmorphine analogue of methadol, and being a methadol it exhibits optical isomerism. The other methadols (acetylmethadol, methadol &c) have at least four optical isomers (see Orlaam).
See also
- Acetylmethadol
- Dimepheptanol (methadol)
References
- ↑ F.. Macdonald. Dictionary of Pharmacological Agents. CRC Press. p. 1447. ISBN 978-0-412-46630-4. Retrieved 11 May 2012.
- ↑ 2.0 2.1 GRUBER CM, BAPTISTI A (1963). "Estimating the acceptability of morphine and noracymethadol in postpartum patients". Clinical Pharmacology and Therapeutics 4: 172–81. PMID 13950878.
- ↑ 3.0 3.1 Lister RE (June 1966). "The toxicity of some of the newer narcotic analgesics". The Journal of Pharmacy and Pharmacology 18 (6): 364–83. doi:10.1111/j.2042-7158.1966.tb07890.x. PMID 4381372.
- ↑ "Controlled Substances in Schedule I". Drug Enforcement Administration - Office of Diversion Control. Retrieved 2012-05-11.
- ↑ Thomas Nordegren (1 March 2002). The A-Z Encyclopedia of Alcohol and Drug Abuse. Universal-Publishers. p. 468. ISBN 978-1-58112-404-0. Retrieved 11 May 2012.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||