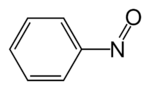
Nitrosobenzene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Nitrosobenzene | |
| Identifiers | |
| 586-96-9 | |
| ChEBI | CHEBI:27986 |
| ChEMBL | ChEMBL98797 |
| ChemSpider | 10989 |
| |
| Jmol-3D images | Image |
| KEGG | C06876 |
| PubChem | 11473 |
| RTECS number | DA6497525 |
| |
| Properties | |
| Molecular formula |
C6H5NO |
| Molar mass | 107.11 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 65 °C (149 °F; 338 K) |
| Boiling point | 59 °C (138 °F; 332 K) (at 18 mmHg) |
| Low | |
| Solubility in other solvents | Sol. in organic solvents |
| Structure | |
| Molecular shape | N is sp2 |
| Hazards | |
| Main hazards | toxic |
| R-phrases | R20/21–R25 |
| S-phrases | S26–S36/37–S45 |
| Related compounds | |
| Related compounds |
Nitrobenzene Aniline |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Nitrosobenzene is the organic compound with the formula C6H5NO. The compound can be viewed as hybrid of singlet O2 and azobenzene. This diamagnetic species exists in equilibrium with its dimer.
Preparation
Nitrosobenzene was first prepared by Adolf von Baeyer by the reaction of diphenylmercury and nitrosyl bromide:[1]
- (C6H5)2Hg + BrNO → C6H5NO + C6H5HgBr
The modern synthesis entails reduction of nitrobenzene to phenylhydroxylamine (C6H5NHOH) which is then oxidized by sodium dichromate (Na2Cr2O7).[2]
Nitrosobenzene can also be prepared by oxidation of aniline using peroxymonosulfuric acid (Caro's acid).[3] It is usually purified by steam distillation, where it comes over as a green liquid that solidifes to a colorless solid.
Characteristic reactions
Nitrosobenzene undergoes Diels–Alder reactions with dienes.[4] Condensation with anilines affords azobenzene derivatives in a reaction known as the Mills reaction.[5] Reduction of nitrosobenzene produces aniline.
Most characteristically, nitrosobenzene condenses with active methylene groups, such as those of malonic esters and benzyl cyanide. For example, condensation with benzylcyanide (PhCH2CN) gives the imine (PhC(CN)=NPh) in a reaction known as the Ehrlich-Sachs Reaction:[6]
- Ph–CH2-CN + Ph–NO → Ph–CH(CN)–N(OH)–Ph (oxyamination adduct) → PhC(CN)=N–Ph
Sometimes condensation with active methylene compounds could give products of O-nitroso-aldol reaction:[7]
- R–CH2-CHO + Ph–NO → R–CH(CHO)–O–NHPh (aminoxylation adduct)
References
- ↑ Baeyer, A. (1874). "Nitrosobenzol und Nitrosonaphtalin". Chemische Berichte 7: 1638–1640. doi:10.1002/cber.187400702214.
- ↑ G. H. Coleman, C. M. McCloskey, F. A. Stuart (1955). "Nitrosobenzene". Org. Synth.; Coll. Vol. 3, p. 668
- ↑ H. Caro (1898). Z. angew. Chem. 11: 845ff. Missing or empty
|title=(help) - ↑ H. Yamamoto, N. Momiyama "Rich Chemistry of Nitroso Compounds" Chemical Communications 2005, pp.3514–25.
- ↑ H. D. Anspon (1955). "p-Phenylazobenzoic Acid". Org. Synth.; Coll. Vol. 3, p. 711
- ↑ H. Feuer. S. Patai, ed. The Chemistry of the Nitro and Nitroso Groups Part 1 (New York: Wiley). pp. 278–283. Missing or empty
|title=(help) - ↑ "Asymmetric O− and N− Nitroso Aldol Reaction – an efficient access to a-oxy and a-amino carbonyl compound" (PDF).