Nitrogen inversion
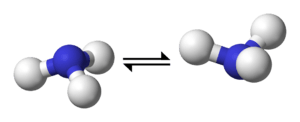 Nitrogen inversion in ammonia | ||
 |
⇌ | 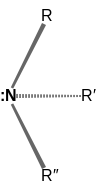 |
| Inversion of an amine. The pair of dots represents the lone electron pair on the nitrogen atom. | ||
In chemistry, a nitrogen compound with a trigonal pyramidal geometry, such as ammonia, undergoes rapid nitrogen inversion, whereby the molecule "turns inside out."[1]
Energy barrier considerations
The ammonia interconversion is rapid at room temperature. Two factors contribute to the rapidity of the inversion: a low energy barrier (24.2 kJ/mol) and a narrow width of the barrier itself, which allows for frequent quantum tunnelling (see below). In contrast, phosphine (PH3) inverts very slowly at room temperature (energy barrier: 132 kJ/mol).[2]
Consequences for optical isomerism
Amines of the type RR′R"N and RR′NH are chiral, but they typically cannot be obtained as individual enantiomers because of the rapidity of the nitrogen inversion. The situation is very different for ammonium salts, RR′R″HN+ and RR′R″R‴N+, and amine oxides, RR′HNO and RR′R″NO, which are optically stable. The corresponding chiral phosphines (RR′R″P and RR′PH), sulfonium salts (RR′R″S+), and sulfoxides (RR′SO) are also optically stable.
Quantum effects
Two aforementioned states of a nitrogen compound, namely ammonia, exhibit a quantum tunnelling because the barrier is narrow.[3] Narrow barriers allow quantum tunnelling, which has nothing to do with thermal excitation. Superposition of two states leads to energy level splitting used in ammonia masers.
Examples
The inversion of ammonia was first detected by microwave spectroscopy in 1934.[4]
In one study the inversion in an aziridine was slowed by a factor of 50 by placing the nitrogen atom in the vicinity of a phenolic alcohol group compared to the oxidized hydroquinone [5]
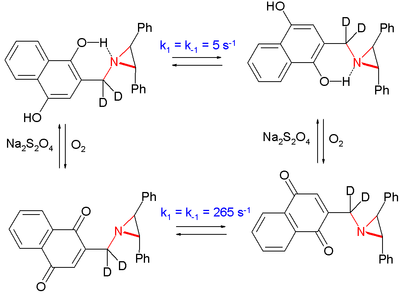
The system interconverts by oxidation by oxygen and reduction by sodium dithionite.
References
- ↑ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ Kölmel, C.; Ochsenfeld, C.; Ahlrichs, R. An ab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines. Theor. Chim. Acta. 1991, 82, 271–284. doi:10.1007/BF01113258
- ↑ Feynman, Richard P.; Robert Leighton; Matthew Sands (1965). "The Hamiltonian matrix". The Feynman Lectures on Physics. Volume III. Massachusetts, USA: Addison-Wesley. ISBN 0-201-02118-8.
- ↑ Cleeton, C.E.; Williams, N.H. (1934). "Electromagnetic waves of 1.1 cm wave-length and the absorption spectrum of ammonia". Physical Reviews 45 (4): 234–237. Bibcode:1934PhRv...45..234C. doi:10.1103/PhysRev.45.234.
- ↑ Control of Pyramidal Inversion Rates by Redox Switching Mark W. Davies, Michael Shipman, James H. R. Tucker, and Tiffany R. Walsh J. Am. Chem. Soc.; 2006; 128(44) pp. 14260–14261; (Communication) doi:10.1021/ja065325f