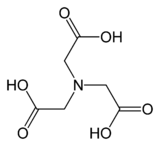
Nitrilotriacetic acid
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′,2′′-Nitrilotriacetic acid | |
| Systematic IUPAC name
2-[Bis(carboxymethyl)amino]acetic acid[1] | |
| Other names
Triglycine[2] | |
| Identifiers | |
| 1710776 | |
| 139-13-9 | |
| ChEBI | CHEBI:44557 |
| ChemSpider | 8428 |
| DrugBank | DB03040 |
| EC number | 205-355-7 |
| 3726 | |
| |
| Jmol-3D images | Image Image |
| KEGG | C14695 |
| MeSH | Nitrilotriacetic+Acid |
| PubChem | 8758 |
| RTECS number | AJ0175000 |
| |
| UN number | 2811 |
| Properties | |
| Molecular formula |
C6H9NO6 |
| Molar mass | 191.14 g·mol−1 |
| Appearance | White crystals |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
−1.3130–−1.3108 MJ mol−1 |
| Hazards | |
| GHS pictograms |   |
| GHS signal word | WARNING |
| H302, H319, H351 | |
| P281, P305+351+338 | |
| EU classification | |
| R-phrases | R22, R36, R40 |
| S-phrases | S26, S36/37 |
| Flash point | 100 °C (212 °F; 373 K) |
| LD50 (Median lethal dose) |
1.1 g kg−1 (oral, rat) |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
|
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid that is used as a chelating agent, which forms coordination compounds with metal ions (chelates) such as Ca2+, Cu2+, and Fe3+.[3]
Production and use
This compound is commercially available as the free acid and as the sodium salt. It is produced from ammonia, formaldehyde, and sodium cyanide or hydrogen cyanide. Worldwide capacity is estimated at 100 thousand tonnes per year.[4]
Coordination chemistry and applications
The uses of NTA are similar to that of EDTA, both being chelating agents. In contrast to EDTA, NTA is easily biodegradable and is almost completely removed during wastewater treatment. It is used for water softening and as a replacement to sodium and potassium triphosphate in detergents, and cleansers.[4] NTA is a tripodal tetradentate trianionic ligand.[5] In the laboratory, this compound is used in complexometric titrations. A variant of NTA is used for protein isolation and purification in the His-tag method. The modified NTA is used to immobilize nickel to a solid support. This allows purification of proteins containing a tag consisting of six histidine residues at either terminus.[6]
- NTA complexes
-
(aq)23views.png)
Three views of the structure of [Ni(NTA)(H2O)2]−.
-
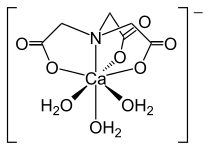
Structure of the [Ca(NTA)(H2O)3]−.
References
- ↑ "Nitrilotriacetic Acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 13 July 2012.
- ↑ Nitrilotriacetic acid
- ↑ NITRILOTRIACETIC ACID AND ITS SALTS, International Agency for Research on Cancer (IARC)
- ↑ 4.0 4.1 Charalampos Gousetis, Hans-Joachim Opgenorth (2005), "Nitrilotriacetic Acid", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a17_377
- ↑ B. L. Barnett, V. A. Uchtman "Structural investigations of calcium-binding molecules. 4. Calcium binding to aminocarboxylates. Crystal structures of Ca(CaEDTA).7H2O and Na(CaNTA)" Inorg. Chem., 1979, volume 18, pp 2674–2678. doi:10.1021/ic50200a007
- ↑ qiaexpressionist