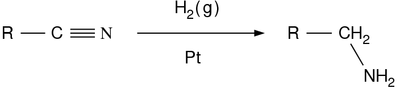Nitrile reduction
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.[1]
Reagents for the conversion to amines are sodium,[2] lithium aluminium hydride, Raney nickel / hydrogen / [3][4][5] or diborane [6] This organic reaction is one of several nitrogen-hydrogen bond forming reactions.
Nitriles can also be reduced to aldehydes. One method is called the Stephen aldehyde synthesis (Tin(II) chloride, hydrochloric acid and hydrolysis of the iminum salt). Aldehydes also form by reduction with hydrogen with in-situ hydrolysis of the imine. Reagents are Raney nickel,[7] lithium aluminium hydride and sodium borohydride.
Nitriles can also be reduced electrochemically[8][9]
References
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ↑ Suter, C. M.; Moffett, Eugene W. (1934). "The Reduction of Aliphatic Cyanides and Oximes with Sodium and n-Butyl Alcohol". Journal of the American Chemical Society 56 (2): 487–487. doi:10.1021/ja01317a502.
- ↑ Organic Syntheses, Coll. Vol. 3, p.229 (1955); Vol. 27, p.18 (1947). Link
- ↑ Organic Syntheses, Coll. Vol. 3, p.358 (1955); Vol. 27, p.33 (1947). Link
- ↑ Organic Syntheses, Coll. Vol. 3, p.720 (1955); Vol. 23, p.71 (1943). Link
- ↑ Organic Syntheses, Coll. Vol. 6, p.223 (1988); Vol. 53, p.21 (1973). Link
- ↑ Organic Syntheses, Coll. Vol. 6, p.631 (1988); Vol. 51, p.20 (1971). Link
- ↑ V. Krishnan, A. Muthukumaran, H. V. K. Udupa (1979). "The electroreduction of benzyl cyanide on iron and cobalt cathodes". Journal of Applied Electrochemistry 9 (5): 657–659. doi:10.1007/BF00610957.
- ↑ V. Krishnan, A. Muthukumaran, H. V. K. Udupa (1983). Process for Electrochemical Preparation of beta phenylethylamine using cobalt black cathode. Calcutta: India Patent Office.