Nitric-oxide synthase (NAD(P)H-dependent)
| Nitric-oxide synthase (NAD(P)H-dependent) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.14.13.165 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Nitric-oxide synthase (NAD(P)H-dependent) (EC 1.14.13.165, nitric oxide synthetase, NO synthase) is an enzyme with system name L-arginine,NAD(P)H:oxygen oxidoreductase (nitric-oxide-forming).[1][2][3] This enzyme catalyses the following chemical reaction
- 2 L-arginine + 3 NAD(P)H + 3 H+ + 4 O2
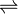 2 L-citrulline + 2 nitric oxide + 3 NAD(P)+ + 4 H2O (overall reaction)
2 L-citrulline + 2 nitric oxide + 3 NAD(P)+ + 4 H2O (overall reaction) - (1a) 2 L-arginine + 2 NAD(P)H + 2 H+ + 2 O2
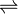 2 Nomega-hydroxy-L-arginine + 2 NAD(P)+ + 2 H2O
2 Nomega-hydroxy-L-arginine + 2 NAD(P)+ + 2 H2O - (1b) 2 Nomega-hydroxy-L-arginine + NAD(P)H + H+ + 2 O2
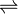 2 L-citrulline + 2 nitric oxide + NAD(P)+ + 2 H2O
2 L-citrulline + 2 nitric oxide + NAD(P)+ + 2 H2O
Nitric-oxide synthase (NAD(P)H-dependent) binds heme (iron protoporphyrin IX) and tetrahydrobiopterin.
See also
References
- ↑ Wang, Z.Q., Lawson, R.J., Buddha, M.R., Wei, C.C., Crane, B.R., Munro, A.W. and Stuehr, D.J. (2007). "Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase". J. Biol. Chem. 282 (4): 2196–2202. doi:10.1074/jbc.M608206200. PMID 17127770.
- ↑ Gusarov, I., Starodubtseva, M., Wang, Z.Q., McQuade, L., Lippard, S.J., Stuehr, D.J. and Nudler, E. (2008). "Bacterial nitric-oxide synthases operate without a dedicated redox partner". J. Biol. Chem. 283: 13140–13147. doi:10.1074/jbc.M710178200. PMC 2442334. PMID 18316370.
- ↑ Agapie, T., Suseno, S., Woodward, J.J., Stoll, S., Britt, R.D. and Marletta, M.A. (2009). "NO formation by a catalytically self-sufficient bacterial nitric oxide synthase from Sorangium cellulosum". Proc. Natl. Acad. Sci. USA 106 (38): 16221–16226. doi:10.1073/pnas.0908443106. PMC 2752531. PMID 19805284.
External links
- Nitric-oxide synthase (NAD(P)H-dependent) at the US National Library of Medicine Medical Subject Headings (MeSH)