Niclosamide
 | |
| Systematic (IUPAC) name | |
|---|---|
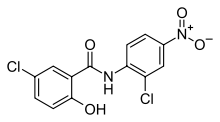
| 5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide | |
| Clinical data | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Identifiers | |
|
50-65-7 | |
| P02DA01 QP52AG03 | |
| PubChem | CID 4477 |
| DrugBank |
DB06803 |
| ChemSpider |
4322 |
| UNII |
8KK8CQ2K8G |
| KEGG |
D00436 |
| ChEMBL |
CHEMBL1448 |
| Chemical data | |
| Formula | C13H8Cl2N2O4 |
| 327.119 g/mol | |
|
SMILES
| |
| |
| | |
Niclosamide (trade name Niclocide) is a teniacide in the anthelmintic family especially effective against cestodes that infect humans and many other animals. It is a salicyanilide compound (5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydrobenzamide) also used as a piscicide. While antihelmintics are a drug family used to treat worm infections, niclosamide is used specifically to treat tapeworms and is not effective against other worms such as pinworms or roundworms. It is a chewable tablet taken orally, dosage depending on type of worm and patient's age and/or weight. It is not usually considered the drug of choice for treating cestode infection in humans or animals because of its side effects, though it remains highly effective and is generally inexpensive.[1]
Along with oxyclozanide, another tapeworm drug, it has been recently found to display "strong in vivo and in vitro activity against Methicillin-resistant Staphylococcus aureus (MRSA)".[2]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medications needed in a basic health system.[3]
Side effects
The medication can have side effects such as abdominal pain, anorexia, diarrhea, and emesis. Rarely, dizziness, skin rash, drowsiness, perianal itching, or an unpleasant taste occur. For some of these reasons, praziquantel is a preferable and equally effective treatment for tapeworm infestation.
Mechanism of action
Niclosamide inhibits glucose uptake, oxidative phosphorylation, and anaerobic metabolism in its target.[4]
References
- ↑ Jim E. Riviere; Mark G. Papich (13 May 2013). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 1096. ISBN 978-1-118-68590-7.
- ↑ Repurposing Salicylanilide Anthelmintic Drugs to Combat Drug Resistant Staphylococcus aureus at PLOS
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Weinbach EC, Garbus J (1969). "Mechanism of action of reagents that uncouple oxidative phosphorylation". Nature 221 (5185): 1016–8. doi:10.1038/2211016a0. PMID 4180173.
- Taber, Clarence Wilbur; Venes, Donald; Thomas, Clayton L. (2001). Taber's cyclopedic medical dictionary. Philadelphia: F.A.Davis Co.
External links
- Niclosamide in the Pesticide Properties DataBase (PPDB)
- Additional Medication Information:Medline
- http://www.drugs.com/cons/Niclosamide.html
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||