Nickel titanium
Nickel titanium, also known as nitinol, is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages e.g. Nitinol 55, Nitinol 60.
| Material properties | |
|---|---|
| Density | 6.45 g/cm3 (0.233 lb/cu in) |
| Electrical Resistivity (Austenite) |
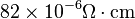 |
| (Martensite) |
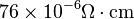 |
| Thermal Conductivity (Austenite) | 0.18 W/cm·K |
| (Martensite) | 0.086 W/cm·K |
| Coefficient of Thermal Expansion (Austenite) |
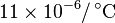 |
| (Martensite) |
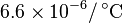 |
| Magnetic Permeability | < 1.002 |
| Magnetic Susceptibility (Austenite) |
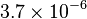 emu/g emu/g |
| (Martensite) |
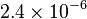 emu/g emu/g |
| Elastic Modulus (Austenite) | 75-83 GPa |
| (Martensite) | 28-40 GPa |
| Yield Strength (Austenite) | 195-690 MPa |
| (Martensite) | 70-140 MPa |
| Poisson's Ratio | 0.33 |
| Nitinol properties are particular to the precise composition of the alloy and its processing. These specifications are typical for commercially available shape memory nitinol alloys. | |
Nitinol alloys exhibit two closely related and unique properties: shape memory and superelasticity (also called pseudoelasticity). Shape memory is the ability of nitinol to undergo deformation at one temperature, then recover its original, undeformed shape upon heating above its "transformation temperature". Superelasticity occurs at a narrow temperature range just above its transformation temperature; in this case, no heating is necessary to cause the undeformed shape to recover, and the material exhibits enormous elasticity, some 10-30 times that of ordinary metal.
History
The term nitinol is derived from its composition and its place of discovery: (Nickel Titanium-Naval Ordnance Laboratory). William J. Buehler[1] along with Frederick Wang,[2] discovered its properties during research at the Naval Ordnance Laboratory in 1959.[3][4] William Buehler was attempting to make a better missile nose cone, which could resist fatigue, heat and the force of impact. Having found that a 1:1 alloy of nickel and titanium could do the job, in 1961 he presented a sample at a laboratory management meeting. The sample, folded up like an accordion, was passed around and flexed by the participants. One of them applied heat from his pipe lighter to the sample and, to everyone's surprise, the accordion-shaped strip stretched and took its previous shape.[5]
While the potential applications for nitinol were realized immediately, practical efforts to commercialize the alloy did not take place until a decade later. This delay was largely because of the extraordinary difficulty of melting, processing and machining the alloy. Even these efforts encountered financial challenges that were not really overcome until the 1990s, when these practical difficulties finally began to be resolved.
The discovery of the shape-memory effect in general dates back to 1932, when Swedish chemist Arne Ölander [6] first observed the property in gold-cadmium alloys. The same effect was observed in Cu-Zn (brass) in the early 1950s.[7]
Mechanism
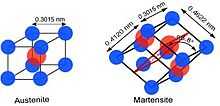
Nitinol's unusual properties are derived from a reversible solid-state phase transformation known as a martensitic transformation, between two different martensite crystal phases, requiring 10,000–20,000 psi (69–138 MPa) of mechanical stress.
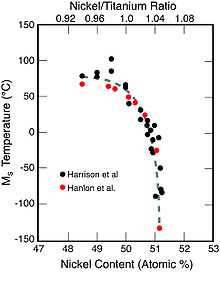
At high temperatures, nitinol assumes an interpenetrating face-centered cubic structure referred to as austenite (also known as the parent phase). At low temperatures, nitinol spontaneously transforms to a more complicated body-centered tetragonal crystal structure known as martensite (daughter phase). The temperature at which austenite transforms to martensite is generally referred to as the transformation temperature. More specifically, there are four transition temperatures. When the alloy is fully austenite, martensite begins to form as the alloy cools at the so-called martensite start, or Ms temperature, and the temperature at which the transformation is complete is called the martensite finish, or Mf temperature. When the alloy is fully martensite and is subjected to heating, austenite starts to form at the As temperature, and finishes at the Af temperature.[8]
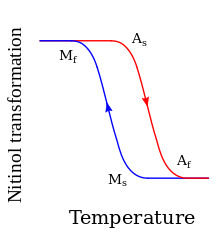
The cooling/heating cycle shows thermal hysteresis. The hysteresis width depends on the precise nitinol composition and processing. Its typical value is a temperature range spanning about 20-50 K (20-50 °C; 36-90 °F).
Crucial to nitinol properties are two key aspects of this phase transformation. First is that the transformation is "reversible", meaning that heating above the transformation temperature will revert the crystal structure to the simpler austenite phase. The second key point is that the transformation in both directions is instantaneous.
Martensite's crystal structure (known as a monoclinic, or B19' structure) has the unique ability to undergo limited deformation in some ways without breaking atomic bonds. This type of deformation is known as twinning, which consists of the rearrangement of atomic planes without causing slip, or permanent deformation. It is able to undergo about 6–8% strain in this manner. When martensite is reverted to austenite by heating, the original austenitic structure is restored, regardless of whether the martensite phase was deformed. Thus the name "shape memory" refers to the fact that the shape of the high temperature austenite phase is "remembered," even though the alloy is severely deformed at a lower temperature.[9]
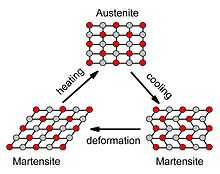
A great deal of force can be produced by preventing the reversion of deformed martensite to austenite — from 35,000 psi to, in many cases, more than 100,000 psi (689 MPa). One of the reasons that nitinol works so hard to return its original shape is that it is not just an ordinary metal alloy, but what is known as an intermetallic compound. In an ordinary alloy, the constituents are randomly positioned on the crystal lattice; in an ordered intermetallic compound, the atoms (in this case, nickel and titanium) have very specific locations in the lattice.[10] The fact that nitinol is an intermetallic is largely responsible for the difficulty in fabricating devices made from the alloy.
The scenario described above (cooling austenite to form martensite, deforming the martensite, then heating to revert to austenite, thus returning the original, undeformed shape) is known as the thermal shape memory effect. To fix the original "parent shape," the alloy must be held in position and heated to about 500 °C (932 °F). A second effect, called superelasticity or pseudoelasticity, is also observed in nitinol. This effect is the direct result of the fact that martensite can be formed by applying a stress as well as by cooling. Thus in a certain temperature range, one can apply a stress to austenite, causing martensite to form while at the same time changing shape. In this case, as soon as the stress is removed, the nitinol will spontaneously return to its original shape. In this mode of use, nitinol behaves like a super spring, possessing an elastic range 10–30 times greater than that of a normal spring material. There are, however, constraints: the effect is only observed about 0-40 K (0-40 °C; 0-72 °F) above the Af temperature.
Nitinol is typically composed of approximately 50 to 51% nickel by atomic percent (55 to 56% weight percent).[10][11] Making small changes in the composition can change the transition temperature of the alloy significantly. One can control the Af temperature in nitinol to some extent, but convenient superelastic temperature ranges are from about −20 °C to +60 °C.
One often-encountered complication regarding nitinol is the so-called R-phase. The R-phase is another martensitic phase that competes with the martensite phase mentioned above. Because it does not offer the large memory effects of the martensite phase, it is, more often than not, an annoyance.
Manufacturing process
Nitinol is exceedingly difficult to make, due to the exceptionally tight compositional control required, and the tremendous reactivity of titanium. Every atom of titanium that combines with oxygen or carbon is an atom that is robbed from the NiTi lattice, thus shifting the composition and making the transformation temperature that much lower. There are two primary melting methods used today:
- Vacuum Arc Remelting: This is done by striking an electrical arc between the raw material and a water-cooled copper strike plate. Melting is done in a high vacuum, and the mold itself is water-cooled copper, so no carbon is introduced during melting.
- Vacuum Induction Melting: This is done by using alternating magnetic fields to heat the raw materials in a crucible (generally carbon). This is also done in a high vacuum, but carbon is introduced during the process.
While both methods have advantages, there are no substantive data showing that material from one process is better than the other. Other methods are also used on a boutique scale, including plasma arc melting, induction skull melting, and e-beam melting. Physical vapour deposition is also used on a laboratory scale.
Hot working of nitinol is relatively easy, but cold working is difficult because the enormous elasticity of the alloy increases die or roll contact, leading to tremendous frictional resistance and tool wear. For similar reasons, machining is extremely difficult—to make things worse, the thermal conductivity of nitinol is poor, so heat is difficult to remove. Grinding (abrasive cutting), Electrical discharge machining (EDM) and laser cutting are all relatively easy.
Heat treating nitinol is delicate and critical. It is the essential tool in fine-tuning the transformation temperature. Aging time and temperature controls the precipitation of various Ni-rich phases, and thus controls how much nickel resides on the NiTi lattice; by depleting the matrix of nickel, aging increases the transformation temperature. The combination of heat treatment and cold working is essential in controlling the properties of nitinol.[12]
Limitations
Fatigue failures of nitinol devices are a constant subject of discussion. Because it is the material of choice for applications requiring enormous flexibility and motion (e.g., peripheral stents and heart valves), it is necessarily exposed to much greater fatigue strains than are other metals. While the strain-controlled fatigue performance of nitinol is superior to all other known metals, fatigue failures have been observed in the most demanding applications. There is a great deal of effort underway trying to better understand and define the durability limits of nitinol.
Nitinol is half nickel, and thus there has been a great deal of concern in the medical industry regarding the release of nickel, a known allergen and possible carcinogen.[12] (Nickel is also present in substantial amounts in stainless steel and cobalt-chrome alloys.) When properly treated (via electropolishing and/or passivation), nitinol forms a very stable protective TiO2 layer that acts as a very effective and self-healing barrier against ion exchange. It has been repeatedly shown that nitinol releases nickel at a slower pace than stainless steel, for example. With that said, very early medical devices were made without electropolishing, and corrosion was observed. Today's nitinol vascular self-expandable metallic stents, for example, show no evidence of corrosion or nickel release, and the outcomes in patients with and without nickel allergies are indistinguishable.
There are constant and long-running discussions regarding inclusions in nitinol, both TiC and Ti2NiOx. All metals contain inclusions, and nitinol cannot be melted without inclusions—they are omnipresent. The size, distribution and type of inclusions can be controlled to some extent. Theoretically, smaller, rounder and few inclusions should lead to increased fatigue durability. All studies done to date, however, have failed to show measurable differences.[13][14]
A major limitation to further use of nitinol has been its difficulty to weld, both to itself and other materials. In the past ten years, laser welding nitinol to itself has become a relatively routine process. More recently, strong joints between NiTi wires and stainless steel wires have been made using nickel filler. More research is ongoing into other processes and other metals to which nitinol can be welded.[15]
Recent advances have shown that processing of Nitinol can expand thermomechanical capabilities, allowing for multiple shape memories to be embedded within a monolithic structure.[16] [17]Research on multi-memory technology is on-going and promises to deliver enhanced shape memory devices in the near future. [18] [19]
Applications
There are four commonly used types of applications for nitinol.
- Free Recovery: nitinol is deformed at a low temperature, and heated to recover its original shape.
- Constrained Recovery: The same, except that recovery is rigidly prevented, and thus a stress is generated.
- Work Production: Here the alloy is allowed to recover, but to do so it must act against a force (thus doing work).
- Superelasticity: As discussed above, here the nitinol acts as a super spring.
In 1989 a survey was conducted in the United States and Canada that involved seven organizations. The survey focused on predicting the future technology, market, and applications of SMAs. The companies predicted the following uses of nitinol in a decreasing order of importance: (1) Couplings, (2) Biomedical and medical, (3) Toys, demonstration, novelty items, (4) Actuators, (5) Heat Engines, (6) Sensors, (7) Cryogenically activated die and bubble memory sockets, and finally (8) lifting devices.[20]
- Nitinol is also popular in extremely resilient glasses frames. It is also used in some mechanical watch springs.
- It can be used as a temperature control system; as it changes shape, it can activate a switch or a variable resistor to control the temperature.
- It is used in cell-phone technology as a retractable antenna, or microphone boom, due to its highly flexible and mechanical memory nature.
- It is used in some novelty products, such as self-bending spoons which can be used by amateur and stage magicians to demonstrate "psychic" powers or as a practical joke, as the spoon will bend itself when used to stir tea, coffee, or any other warm liquid.
- It can also be used as wires which are used to locate and mark breast tumours so that the following surgery can be more exact.
- Due to the fact it can change shapes it is also used as a golf club insert.
- Nickel titanium can be used to make the underwires for underwire bras.[21][22][23]
Heat engines
- Demonstration model heat engines have been built which use nitinol wire to produce mechanical energy from hot and cold heat sources.[24] A prototype commercial engine developed in the 1970s by engineer Ridgway Banks at Lawrence Berkeley National Laboratory, was named the Banks Engine.[25][26][27][28][29]
Biocompatible applications
- Nitinol is highly biocompatible and has properties suitable for use in orthopaedic implants. Due to Nitinol's unique properties it has seen a large demand for use in less invasive medical devices. Nitinol tubing is commonly used in catheters, stents, and superelastic needles.
- In colorectal surgery , the material is used in devices for reconnecting the intestine after removing the pathology.
- Nitinol is used for devices developed by Franz Freudenthal to treat Patent ductus arteriosus, blocking a blood vessel that bypasses the lungs and has failed to close after birth in an infant.[30]
- In dentistry, the material is used in orthodontics for brackets and wires connecting the teeth. Once the SMA wire is placed in the mouth its temperature rises to ambient body temperature. This causes the nitinol to contract back to its original shape, applying a constant force to move the teeth. These SMA wires do not need to be retightened as often as other wires because they can contract as the teeth move unlike conventional stainless steel wires. Additionally, nitinol can be used in endodontics, where nitinol files are used to clean and shape the root canals during the root canal procedure. Because of the high fatigue tolerance and flexibility of nitinol, it greatly decreases the possibility of an endodontic file breaking inside the tooth during root canal treatment, thus improving safety for the patient.
- Another significant application of nitinol in medicine is in stents: a collapsed stent can be inserted into an artery or vein, where body temperature warms the stent and the stent returns to its original expanded shape following removal of a constraining sheath; the stent then helps support the artery or vein to improve blood flow. It is also used as a replacement for sutures - nitinol wire can be weaved through two structures then allowed to transform into its preformed shape, which should hold the structures in place.
References
- ↑ Buehler, W. J.; Gilfrich, J. W.; Wiley, R. C. (1963). "Effects of Low-Temperature Phase Changes on the Mechanical Properties of Alloys Near Composition TiNi". Journal of Applied Physics 34 (5): 1475–1477. doi:10.1063/1.1729603.
- ↑ Wang, F. E.; Buehler, W. J.; Pickart, S. J. (1965). "Crystal Structure and a Unique Martensitic Transition of TiNi". Journal of Applied Physics 36 (10): 3232–3239. doi:10.1063/1.1702955.
- ↑ "The Alloy That Remembers", Time, 1968-09-13
- ↑ Kauffman, G. B.; Mayo, I. (1997). "The Story of Nitinol: The Serendipitous Discovery of the Memory Metal and Its Applications". The Chemical Educator 2 (2): 1–21. doi:10.1007/s00897970111a.
- ↑ Withers, N. (2014). "Magnificent Molecules - Nitinol" (PDF). The Mole (RSC) 2014 (2): 4.
- ↑ Ölander, A. (1932). "An Electrochemical Investigation of Solid Cadmium-Gold Alloys". Journal of the American Chemical Society 54 (10): 3819–3833. doi:10.1021/ja01349a004.
- ↑ Hornbogen, E.; Wassermann, G. (1956). "Über den Einfluβ von Spannungen und das Auftreten von Umwandlungsplastizität bei β1-β-Umwandlung des Messings". Zeitschrift für Metallkunde 47: 427–433.
- ↑ "Nitinol facts". Nitinol.com. 2013.
- ↑ Funakubo, Hiroyasu (1984), Shape memory alloys, University of Tokyo, pp. 7, 176.
- ↑ 10.0 10.1 "Nitinol SM495 Wire" (PROPERTIES, PDF). 2013.
- ↑ "Nitinol SE508 Wire" (PROPERTIES, PDF). 2013.
- ↑ 12.0 12.1 Pelton, A.; Russell, S.; DiCello, J. (2003). "The Physical Metallurgy of Nitinol for Medical Applications". JOM 55 (5): 33–37. doi:10.1007/s11837-003-0243-3.
- ↑ Morgan, N.; Wick, A.; DiCello, J.; Graham, R. (2006). "Carbon and Oxygen Levels in Nitinol Alloys and the Implications for Medical Device Manufacture and Durability". SMST-2006 Proceedings of the International Conference on Shape Memory and Superelastic Technologies (PDF). ASM International. p. 821–828. doi:10.1361/cp2006smst821. ISBN 978-0-87170-862-5. LCCN 2009499204.
- ↑ Miyazaki, S.; Sugaya, Y.; Otsuka, K. (1989). "Mechanism of Fatigue Crack Nucleation in Ti-Ni Alloys". Shape memory materials : May 31-June 3, 1988, Sunshine City, Ikebukuro, Tokyo, Japan. Proceedings of the MRS International Meeting on Advanced Materials 9. Materials Research Society. p. 257–262. ISBN 1-55899-038-0. LCCN 90174266.
- ↑ US patent 6875949, Hall, P. C., "Method of Welding Titanium and Titanium Based Alloys to Ferrous Metals"
- ↑ Khan, M. I.; Zhou Y. N. (2011), Methods and Systems for Processing Materials, Including Shape Memory Materials, WO Patent WO/2011/014,962
- ↑ Daly, M.; Pequegnat, A.; Zhou, Y.; Khan, M. I. (2012), "Enhanced thermomechanical functionality of a laser processed hybrid NiTi–NiTiCu shape memory alloy", Smart Materials and Structures 21 (4): 045018, doi:10.1088/0964-1726/21/4/045018
- ↑ Daly, M.; Pequegnat, A.; Zhou, Y. N.; Khan, M. I. (2012), "Fabrication of a novel laser-processed NiTi shape memory microgripper with enhanced thermomechanical functionality", Journal of Intelligent Material Systems and Structures 24 (8): 984–990, doi:10.1177/1045389X12444492
- ↑ Pequegnat, A.; Daly, M.; Wang, J.; Zhou, Y.; Khan, M. I. (2012), "Dynamic actuation of a novel laser-processed NiTi linear actuator", Smart Materials and Structures 21 (9): 094004, doi:10.1088/0964-1726/21/9/094004
- ↑ Miller, R. K.; Walker, T. (1989). Survey on Shape Memory Alloys. Survey Reports 89. Future Technology Surveys. p. 17. ISBN 9781558651005. OCLC 38076438.
- ↑ Brady, G. S.; Clauser, H. R.; Vaccari, J. A. (2002). Materials Handbook (15th ed.). McGraw-Hill Professional. p. 633. ISBN 978-0-07-136076-0. Retrieved 2009-05-09.
- ↑ Sang, D.; Ellis, P.; Ryan, L.; Taylor, J.; McMonagle, D.; Petheram, L.; Godding, P. (2005). Scientifica. Nelson Thornes. p. 80. ISBN 0-7487-7996-5. Retrieved 2009-05-09.
- ↑ Jones, G.; Falvo, M. R.; Taylor, A. R.; Broadwell, B. P. (2007). "Nanomaterials: Memory Wire". Nanoscale Science. NSTA Press. p. 109. ISBN 1-933531-05-3. Retrieved 2009-05-09.
- ↑ "Nitinol Heat Engine Kit". Images Scientific Instruments. 2007. Retrieved 2011.
- ↑ Banks, R. (1975). "The Banks Engine". Die Naturwissenschaften 62 (7): 305–308. doi:10.1007/BF00608890.
- ↑ Vimeo posting of "The Individualist", documentary on Ridgway Banks
- ↑ "Single wire nitinol engine", Ridgway M. Banks, US Patent
- ↑ "Metals that Remember", Popular Science, January 1988
- ↑ "Engine Uses No Fuel", Milwaukee Journal, December 5, 1973
- ↑ Alejandra Martins (2014-10-02). "The inventions of the Bolivian doctor who saved thousands of children". BBC Mundo. Retrieved 2015-03-30.
Further reading
- H.R. Chen, ed., Shape Memory Alloys: Manufacture, Properties and Applications, Nova Science Publishers Inc., 2010, ISBN 978-1-60741-789-7.
- Y.Y. Chu & L.C. Zhao, eds., Shape Memory Materials and Its [sic] Applications, Trans Tech Publications Ltd., 2002, ISBN 0-87849-896-6.
- D.C. Lagoudas, ed., Shape Memory Alloys, Springer Science+Business Media LLC, 2008, ISBN 978-0-387-47684-1.
- K. Ōtsuka & C.M. Wayman, eds., Shape Memory Materials, Cambridge University Press, 1998, ISBN 0-521-44487-X
- Sai V. Raj, Low Temperature Creep of Hot-extruded Near-stoichiometric NiTi Shape Memory Alloy, National Aeronautics and Space Administration, Glenn Research Center, 2013.
- Gerald Julien, Nitinol Technologies,Inc Edgewood, Wa. Us patent" 6422010 Manufacturing of Nitinol Parts & Forms
A process of making parts and forms of Type 60 Nitinol having a shape memory effect, comprising: selecting a Type 60 Nitinol. Inventor G,Julien CEO of Nitinol Technologies, Inc. (Washington State)
External links
| Wikimedia Commons has media related to Nickel titanium. |
- Society of Shape Memory and Superelastic Technologies
- Nitinol Resource Library
- Physical properties of nitinol
- Nitinol Technical Resource Library
- Literature on Nitinol Wire
- Nitinol-Tubing
Science Digest articles - Miracle Metal 1982 - PDF