Nickel(II) chloride
-chloride-hexahydrate-sample.jpg) Hexahydrate | |
-chloride.jpg) Anhydrous | |
| Names | |
|---|---|
| IUPAC name
Nickel(II) chloride | |
| Other names
Nickelous chloride, nickel(II) salt of hydrochloric acid | |
| Identifiers | |
| 7718-54-9 7791-20-0 (hexahydrate) | |
| ChEBI | CHEBI:34887 |
| ChemSpider | 22796 |
| EC number | 231-743-0 |
| |
| Jmol-3D images | Image |
| KEGG | C14711 |
| PubChem | 24385 |
| RTECS number | QR6480000 |
| |
| Properties | |
| NiCl2 | |
| Molar mass | 129.5994 g/mol (anhydrous) 237.69 g/mol (hexahydrate) |
| Appearance | yellow-brown crystals deliquescent (anhydrous) green crystals (hexahydrate) |
| Odor | odorless |
| Density | 3.55 g/cm3 (anhydrous) 1.92 g/cm3 (hexahydrate) |
| Melting point | 1,001 °C (1,834 °F; 1,274 K) (anhydrous) 140 °C (hexahydrate) |
| anhydrous 64.2 g/100 mL (20 °C) 87.6 g/100 mL (100 °C) hexahydrate 254 g/100 mL (20 °C) 600 g/100 mL (100 °C) | |
| Solubility | 0.8 g/100 mL (hydrazine) soluble in ethylene glycol, ethanol, ammonium hydroxide insoluble in ammonia, nitric acid |
| Acidity (pKa) | 4 (hexahydrate) |
| Structure | |
| Crystal structure | Monoclinic |
| octahedral at Ni | |
| Thermochemistry | |
| Std molar entropy (S |
107 J·mol−1·K−1[1] |
| Std enthalpy of formation (ΔfH |
−316 kJ·mol−1[1] |
| Hazards | |
| MSDS | Fischer Scientific |
| EU Index | 028-011-00-6 |
| EU classification | Carc. Cat. 1 Muta. Cat. 3 Repr. Cat. 2 Toxic (T) Irritant (Xi) Dangerous for the environment (N) |
| R-phrases | R49, R61, R23/25, R38, R42/43, R48/23, R68, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions |
Nickel(II) fluoride Nickel(II) bromide Nickel(II) iodide |
| Other cations |
Palladium(II) chloride Platinum(II) chloride Platinum(II,IV) chloride Platinum(IV) chloride |
| Related compounds |
Cobalt(II) chloride Copper(II) chloride |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Nickel(II) chloride (or just nickel chloride), is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. In general nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts are carcinogenic.
Production and syntheses
The largest scale production of nickel chloride involves the extraction with hydrochloric acid of nickel matte and residues obtained from roasting refining nickel-containing ores.
NiCl2·6H2O is rarely prepared in the laboratory because it is inexpensive and has a long shelf-life. Heating the hexahydrate in the range 66-133.°C gives the yellowish dihydrate, NiCl2·2H2O.[2] The hydrates convert to the anhydrous form upon heating in thionyl chloride or by heating under a stream of HCl gas. Simply heating the hydrates does not afford the anhydrous dichloride.
- NiCl2·6H2O + 6 SOCl2 → NiCl2 + 6 SO2 + 12 HCl
The dehydration is accompanied by a color change from green to yellow.[3]
Structure of NiCl2 and its hydrates
NiCl2 adopts the CdCl2 structure.[4] In this motif, each Ni2+ center is coordinated to six Cl− centers, and each chloride is bonded to three Ni(II) centers. In NiCl2 the Ni-Cl bonds have "ionic character". Yellow NiBr2 and black NiI2 adopt similar structures, but with a different packing of the halides, adopting the CdI2 motif.
In contrast, NiCl2·6H2O consists of separated trans-[NiCl2(H2O)4] molecules linked more weakly to adjacent water molecules. Only four of the six water molecules in the formula are bound to the nickel, and the remaining two are water of crystallisation.[4] Cobalt(II) chloride hexahydrate has a similar structure. The hexahydrate occurs in nature as the very rare mineral nickelbischofite.
The dihydrate NiCl2·2H2O adopts a structure intermediate between the hexahydrate and the anhydrous forms. It consists of infinite chains of NiCl2, wherein both chloride centers are bridging ligands. The trans sites on the octahedral centers occupied by aquo ligands.[5] A tetrahydrate NiCl2·4H2O is also known.
Reactions
Nickel(II) chloride solutions are acidic, with a pH of around 4 due to the hydrolysis of the Ni2+ ion.
Coordination complexes
_complexes_in_aqueous_solution.jpg)
Most of the reactions ascribed to "nickel chloride" involve the hexahydrate, although specialized reactions require the anhydrous form.
Reactions starting from NiCl2·6H2O can be used to form a variety of nickel coordination complexes because the H2O ligands are rapidly displaced by ammonia, amines, thioethers, thiolates, and organophosphines. In some derivatives, the chloride remains within the coordination sphere, whereas chloride is displaced with highly basic ligands. Illustrative complexes include:
| Complex | Color | Magnetism | Geometry |
|---|---|---|---|
| [Ni(NH3)6]Cl2 | blue/violet | paramagnetic | octahedral |
| [Ni(en)3]2+ | violet | paramagnetic | octahedral |
| NiCl2(dppe) | orange | diamagnetic | square planar |
| [Ni(CN)4]2− | colorless | diamagnetic | square planar |
| [NiCl4]2−[6][7] | Yellowish-green | paramagnetic | tetrahedral |
Some nickel chloride complexes exist as an equilibrium mixture of two geometries; these examples are some of the most dramatic illustrations of structural isomerism for a given coordination number. For example, NiCl2(PPh3)2, containing four-coordinate Ni(II), exists in solution as a mixture of both the diamagnetic square planar and the paramagnetic tetrahedral isomers. Square planar complexes of nickel can often form five-coordinate adducts.
NiCl2 is the precursor to acetylacetonate complexes Ni(acac)2(H2O)2 and the benzene-soluble (Ni(acac)2)3, which is a precursor to Ni(1,5-cyclooctadiene)2, an important reagent in organonickel chemistry.
In the presence of water scavengers, hydrated nickel(II) chloride reacts with dimethoxyethane (dme) to form the molecular complex NiCl2(dme)2. The dme ligands in this complex are labile. For example, this complex reacts with sodium cyclopentadienide to give the sandwich compound nickelocene.
Applications in organic synthesis
NiCl2 and its hydrate are occasionally useful in organic synthesis.[8]
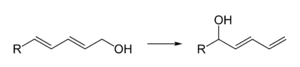
- As a mild Lewis acid, e.g. for the regioselective isomerization of dienols:
- In combination with CrCl2 for the coupling of an aldehyde and a vinylic iodide to give allylic alcohols.
- For selective reductions in the presence of LiAlH4, e.g. for the conversion of alkenes to alkanes.
- As a precursor to nickel boride, prepared in situ from NiCl2 and NaBH4. This reagent behaves like Raney Nickel, comprising an efficient system for hydrogenation of unsaturated carbonyl compounds.
- As a precursor to finely divided Ni by reduction with Zn, for the reduction of aldehydes, alkenes, and nitro aromatic compounds. This reagent also promotes homo-coupling reactions, that is 2RX → R-R where R = aryl, vinyl.
- As a catalyst for making dialkyl arylphosphonates from phosphites and aryl iodide, ArI:
- ArI + P(OEt)3 → ArP(O)(OEt)2 + EtI
Other uses
Nickel chloride solutions are used for electroplating nickel onto other metal items.
Safety
Nickel(II) chloride is irritating upon ingestion, inhalation, skin contact, and eye contact. Prolonged exposure to nickel and its compounds have been shown to produce cancer.
References
- ↑ 1.0 1.1 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 0-618-94690-X.
- ↑ Laird G. L. Ward "Anhydrous Nickel (II) Halides and their Tetrakis(Ethanol) and 1,2-Dimethoxyethane Complexes Inorganic Syntheses, 1972 Volume 13, pages 154–164. doi:10.1002/9780470132449.ch30
- ↑ Pray, A. P.; Tyree, S. Y.; Martin, Dean F.; Cook, James R. (1990). "Anhydrous Metal Chlorides". Inorganic Syntheses 28: 321–2. doi:10.1002/9780470132593.ch80.
- ↑ 4.0 4.1 , Wells, A. F. Structural Inorganic Chemistry, Oxford Press, Oxford, United Kingdom, 1984.
- ↑ B. Morosin "An X-ray diffraction study on nickel(II) chloride dihydrate" Acta Cryst. 1967. volume 23, pp. 630-634. doi:10.1107/S0365110X67003305
- ↑ Gill, N. S. and Taylor, F. B. (1967). "Tetrahalo Complexes of Dipositive Metals in the First Transition Series". Inorganic Syntheses 9: 136–142. doi:10.1002/9780470132401.ch37.
- ↑ G. D. Stucky, J. B. Folkers, T. J. Kistenmacher (1967). "The Crystal and Molecular Structure of Tetraethylammonium Tetrachloronickelate(II)". Acta Crystallographica 23 (6): 1064–1070. doi:10.1107/S0365110X67004268.
- ↑ Tien-Yau Luh, Yu-Tsai Hsieh Nickel(II) Chloride" in Encyclopedia of Reagents for Organic Synthesis (L. A. Paquette, Ed.) 2001 J. Wiley & Sons, New York. doi:10.1002/047084289X.rn012. Article Online Posting Date: April 15, 2001.
External links
| Wikimedia Commons has media related to Nickel(II) chloride. |
- NIOSH Pocket Guide to Chemical Hazards
- Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov
| ||||||||||||||||||