Nerve growth factor
Nerve growth factor (NGF) is a small secreted protein that is important for the growth, maintenance, and survival of certain target neurons (nerve cells). It also functions as a signaling molecule.[1][2] It is perhaps the prototypical growth factor, in that it is one of the first to be described. While "nerve growth factor" refers to a single factor,[3] "nerve growth factors" refers to a family of factors also known as neurotrophins.[4] Other members of the neurotrophin family that are well recognized include Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3), and Neurotrophin 4/5 (NT-4/5).
Function and mechanism of action
NGF is critical for the survival and maintenance of sympathetic and sensory neurons, as they will undergo apoptosis in the absence of the protein.[5] Nerve growth factor causes axonal growth. Studies have shown that it causes axonal branching and a bit of elongation.[6] NGF binds with at least two classes of receptors: the p75 LNGFR (for "low-affinity nerve growth factor receptor") neurotrophin receptor (p75(NTR)) and TrkA, a transmembrane tyrosine kinase. Both are associated with neurodegenerative disorders.
NGF binds to high-affinity tropomyosin receptor kinase A (TrkA). TrkA dimerizes and autophosphorylates its tyrosine kinase segment, which leads to the activation of PI 3-kinase, ras, and PLC signaling pathways. Alternatively, the p75NTR receptor can form a heterodimer with TrkA which has higher affinity and specificity for NGF.
There is evidence that NGF circulates throughout the entire body and is important for maintaining homeostasis.[7]
Major neuron-survival pathways mediated by NGF signaling
Binding interaction between NGF and the TrkA receptor facilitates receptor dimerization and tyrosine residue phosphorylation of the cytoplasmic tail by adjacent Trk receptors.[8] Trk receptor phosphorylation sites operate as Shc adaptor protein docking sites, which undergo phosphorylation by the TrkA receptor[9] Once the cytoplasmic adaptor protein (Shc) is phosphorylated by the receptor cytoplasmic tail, cell survival is initiated through several intracellular pathways.
One major pathway leads to the activation of the serine/threonine kinase, Akt. This pathway begins with the Trk receptor complex-recruitment of a second adaptor protein called growth factor-receptor bound protein-2 (Grb2) along with a docking protein called Grb2-associated Binder-1 (GAB1).[9] Subsequently, phosphatidylinositol-3 kinase (PI3K) is activated, resulting in Akt kinase activation.[9] Study results have shown that blocking PI3K or Akt activity results in death of sympathetic neurons in culture, regardless of NGF presence.[10] However if either kinase is constitutively active, neurons survive even without NGF.[10]
A second pathway contributing to cell survival occurs through activation of the mitogen-activated protein kinase (MAPK) kinase. In this pathway, recruitment of a guanine nucleotide exchange factor by the adaptor and docking proteins leads to activation of a membrane-associated G-protein known as Ras.[9] The guanine nucleotide exchange factor mediates Ras activation through the GDP-GTP exchange process. The active Ras protein phosphorylates several proteins, along with the serine/ threonine kinase, Raf.[9] Raf, in turn activates the MAPK cascade to facilitate ribosomal s6 kinase(RSK) activation and transcriptional regulation.[9]
Both Akt and RSK, components of the PI3K-Akt and MAPK pathways respectively, act to phosphorylate the cyclic AMP response element binding protein (CREB) transcription factor.[9] Phosphorylated CREB translocates into the nucleus and mediates increased expression of anti-apoptotic proteins,[9] thus promoting NGF-mediated cell survival. However, in the absence of NGF, the expression of pro-apoptotic proteins is increased when the activation of cell death-promoting transcription factors such as c-Jun are not suppressed by the aforementioned NGF-mediated cell survival pathways.[9]
Roles of ProNGF in the survival and death of neurons
There is also evidence that shows that the precursor to NGF, pro-NGF, may also play important roles due to its abundance. These include apoptotic and neurotrophic properties.[11]
Pro-NGF is the uncleaved, precursor protein form of the active peptide form of NGF. The Pro-NGF precursor is biologically inactive, as it does not undergo post-transcriptional modification. Pro-NGF acts with a coreceptor, sortilin, to bind the 75kD neurotrophin receptor known as P75NTR (a tumor necrosis factor family member).[9] High affinity binding between Pro-NGF, sortilin, and p75NTR can result in either survival or programmed cell death (PCD). Study results indicate that superior cervical ganglia neurons that express both p75NTR and TrkA die when treated with proNGF,[12] where as NGF treatment of these same neurons results in survival and axonal growth. Survival and PCD mechanisms are mediated through adaptor protein binding to the death domain of the p75NTR cytoplasmic tail. Survival occurs when recruited cytoplasmic adaptor proteins facilitate signal transduction through tumor necrosis factor receptor members such as TRAF6, which results in the release of nuclear factor κB (NF-κB) transcription activator.[9] NF-κB regulates nuclear gene transcription to promote cell survival. Alternatively, PCD occurs when TRAF6 and neurotrophin receptor interacting factor (NRIF) are both recruited to activate c-Jun N-terminal kinase (JNK); which phosphorylates c-Jun. The activated transcription factor c-Jun regulates nuclear transcription to increase pro-apoptotic gene transcription.[9]
Ovulation
NGF is abundant in seminal plasma. Recent studies have found that it induces ovulation in some mammals e.g. “induced” ovulators, such as llamas. Surprisingly, research showed that these induced animals will also ovulate when semen from on-schedule or “spontaneous” ovulators, such as cattle is used. Its significance in humans is currently unknown. It was previously dubbed ovulation-inducing factor (OIF) in semen before its final identification as β-NGF in 2012.[13]
Structure
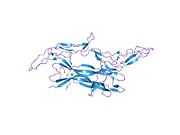
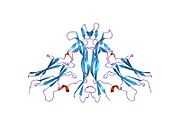
The structure of NGF was first solved by X-ray crystallography and published in 1991 by McDonald et al. in Nature.[14][15] NGF forms a cystine knot structure made up of beta strands twisted around each other and linked by disulfide bonds. Most structures are dimeric. At the time this structure was solved, this fold had never been seen before. Hence NGF is the founding member of the nerve growth factor family of structurally conserved proteins.
History
Rita Levi-Montalcini and Stanley Cohen discovered NGF in the 1950s while faculty members at Washington University in St Louis. However, its discovery, along with the discovery of other neurotrophins, was not widely recognized until 1986, when it won the Nobel Prize in Physiology or Medicine.[16][17][18]
Studies in 1971 determined the primary structure of NGF. This eventually led to the discovery of the NGF gene.
NGF is abundant in seminal plasma. Recent studies have found that it induces ovulation in some mammals.[19]
Clinical significance
NGF prevents or reduces neuronal degeneration in animal models of neurodegenerative diseases and these encouraging results in animals have led to several clinical trials in humans.[20] NGF has also been shown to promote peripheral nerve regeneration in rats.[21] The expression of NGF is increased in inflammatory diseases where it suppresses inflammation.[22] Also, NGF appears to promote myelin repair.[23] Hence NGF may be useful for the treatment of multiple sclerosis.[24] NGF could also be involved in various psychiatric disorders, such as dementia, depression, schizophrenia, autism, Rett syndrome, anorexia nervosa, and bulimia nervosa.[25] Dysregulation of NGF signaling has also been linked to Alzheimer's disease.[26][27][28][29][30][31]
Neurotrophins, including NGF, have been shown to affect many areas of the brain, including areas that are related to Rett syndrome, bipolar disorder, and Alzheimer’s disease. Stress and/or anxiety are usually a precipitating factor in these disorders and affects levels of NGF, leading to impaired cognitive functioning.
This impaired cognitive functioning can be seen in patients with Schizophrenia. In treatment of schizophrenia, NGF levels are increased in patients using atypical antipsychotic medication, but not in patients using typical antipsychotic medications. Patients using atypical medications usually report improved cognitive performance compared to those using typical antipsychotics. In addition, these higher NGF levels from the atypical antipsychotic medications lead to a reduction in negative symptoms of Schizophrenia.[32]
Nerve Growth Factor has been shown to restore learning ability in rats recovering from induced aloholism[33]
Rett syndrome and Autism often show similar signs early in life, such as slowing development and intellectual disability. One distinguishing factor is that low levels of NGF have been found in the cerebral spinal fluid of those with Rett Syndrome compared to children with Autism who have relatively normal to high levels[34] Pharmaceutical therapies with NGF-like activity can be effective in treating Rett syndrome, including better motor and cortical functioning as well as increased social communication.[35]
Impairment of neuroplasticity and altered levels of neuro-trophins are involved in Bipolar Disorder. NGF has been found to be decreased overall in Bipolar Disorder patients. More specifically, while in a manic state NGF is especially low. This leads to elevated or irritable mood with increased energy and decreased need for sleep while in a manic state. This decreased NGF may serve as a biological marker when assessing the present state of a Bipolar Disorder patient.[36] When Bipolar Disorder patients were treated with lithium, their NGF concentrations increased in the frontal cortex, limbic forebrain, hippocampus, and amygdala.[37]
An increase in cortical and subcortical NGF has been found in patients with Alzheimer’s disease. Alzheimer’s is a neurodegenerative disease with which dysregulation of NGF signaling has also been linked, causing impaired retrograde transport of NGF to certain areas of the brain. This impairment may be caused by an atypical production or use of receptors in the brain.[38] Stimulating NGF receptors via NGF infusion has been shown to increase blood flow and verbal episodic memory. These improvements have been longer lasting than other treatments for Alzheimer’s.[35]
Also, NGF has been shown to play a role in number cardiovascular diseases, such as coronary atherosclerosis, obesity, type 2 diabetes, and metabolic syndrome.[39] Reduced plasma levels of NGF and BDNF have been associated with acute coronary syndromes and metabolic syndromes.[40][41] NGF is known to have insulinotropic, angiogenic, and antioxidant properties. NGF suppresses food intake.
NGF has also been shown to accelerate wound healing. There is evidence that it could be useful in the treatment of skin ulcers and cornea ulcers.[42]
In some gynecological diseases, an elevated prostaglandin E2 is thought to stimulate production of NGF which contributes to the perception of pain and increased inflammation in endometriosis.[43]
Monoclonal antibodies against NGF have been used in clinical trials to modulate pain. One of these is Tanezumab.
Cultural significance
In 2005, Enzo Emanuele and coworkers at University of Pavia found that nerve growth factor (NGF) has high levels when people first fall in love, but these levels return to as they were after one year. To be specific, four neurotrophin levels, i.e., NGF, BDNF, NT-3, and NT-4, of 58 subjects who had recently fallen in love were compared with levels in a control group who were either single or already engaged in a long-term relationship. The results showed that NGF levels were significantly higher in the subjects in love than as compared to either of the control groups.[44][45][46] Nerve growth factor may contribute to increased longevity and mental capacity.[47] Centenarian Rita Levi-Montalcini took a daily solution in the form of eye drops, and has stated that her brain is more active now than it was four decades ago.[47] In 2014, Sundaravadivel Balasubramanian and coworkers at Medical University of South Carolina showed that NGF level is elevated in people who performed a single session 20 minute Yoga Breathing involving Om chanting and Thirumoolar Pranayama, when compared to Control group.[48]
Interactions
Nerve growth factor has been shown to interact with TrkA[12][49][50] and Low affinity nerve growth factor receptor.[12][49]
See also
- Protein targeting
- Nervous System
- VGF Nerve Growth Factor-inducible, a protein whose expression is induced by NGF
- neurotrophin
- growth factor
- brain-derived neurotrophic factor
- neurotrophin-3
- neurotrophin-4
- nerve growth factor receptor
- Hericium erinaceus an edible mushroom that has been shown to boost NGF
- Huperzine A an herb-derived alkaloid that seems to boost NGF
- Polygala tenuifolia a Chinese herb shown to increase NGF secretion in astrocytes
- Therapygenetics - showing how NGF genes predict treatment outcome to cognitive behavioural therapy
References
- ↑ Fiore M, Chaldakov GN, Aloe L (2009). "Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems". Rev Neurosci 20 (2): 133–45. doi:10.1515/revneuro.2009.20.2.133. PMID 19774790.
- ↑ Purves D, Augustine G, Fitzpatrick D, Hall W, LaMantia A, McNamara J, White L (2004). Neuroscience. Sunderland, Mass: Sinauer. pp. 72–173, 600–606. ISBN 0-87893-725-0.
- ↑ Nerve Growth Factor at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Nerve Growth Factors at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Freeman RS, Burch RL, Crowder RJ, Lomb DJ, Schoell MC, Straub JA et al. (2004). "NGF deprivation-induced gene expression: after ten years, where do we stand?". Prog. Brain Res. Progress in Brain Research 146: 111–26. doi:10.1016/S0079-6123(03)46008-1. ISBN 978-0-444-51472-1. PMID 14699960.
- ↑ Madduri S, Papaloïzos M, Gander B (September 2009). "Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro". Neurosci. Res. 65 (1): 88–97. doi:10.1016/j.neures.2009.06.003. PMID 19523996.
- ↑ Levi-Montalcini R (2004). "The nerve growth factor and the neuroscience chess board". Prog. Brain Res. 146: 525–7. doi:10.1016/s0079-6123(03)46033-0. PMID 14699984.
- ↑ Kaplan DR, Martin-Zanca D, Parada LF (March 1991). "Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF". Nature 350 (6314): 158–60. doi:10.1038/350158a0. PMID 1706478.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 9.9 9.10 9.11 Sanes DH, Thomas AR, Harris WA (2011). "Naturally-occurring neuron death". Development of the Nervous System, Third Edition. Boston: Academic Press. pp. 171–208. ISBN 0-12-374539-X.
- ↑ 10.0 10.1 Crowder RJ, Freeman RS (April 1998). "Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons". J. Neurosci. 18 (8): 2933–43. PMID 9526010.
- ↑ Fahnestock M, Yu G, Coughlin MD (2004). "ProNGF: a neurotrophic or an apoptotic molecule?". Prog. Brain Res. 146: 101–10. doi:10.1016/S0079-6123(03)46007-X. PMID 14699959.
- ↑ 12.0 12.1 12.2 Lee R, Kermani P, Teng KK, Hempstead BL (November 2001). "Regulation of cell survival by secreted proneurotrophins". Science 294 (5548): 1945–8. doi:10.1126/science.1065057. PMID 11729324.
- ↑ Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA et al. (September 2012). "The nerve of ovulation-inducing factor in semen". Proc. Natl. Acad. Sci. U.S.A. 109 (37): 15042–7. doi:10.1073/pnas.1206273109. PMC 3443178. PMID 22908303. Lay summary – sciencenews.org.
- ↑ PDB 1bet; McDonald NQ, Lapatto R, Murray-Rust J, Gunning J, Wlodawer A, Blundell TL (December 1991). "New protein fold revealed by a 2.3-A resolution crystal structure of nerve growth factor". Nature 354 (6352): 411–4. doi:10.1038/354411a0. PMID 1956407.
- ↑ "NGF - twenty years a-growing". Quips. Protein Data Bank Europe.
- ↑ The 1986 Nobel Prize in Physiology or Medicine for discoveries of growth factors
- ↑ Presentation Speech by Professor Kerstin Hall The Nobel Prize in Physiology or Medicine 1986
- ↑ Rita Levi-Montalcini – Nobel Lecture
- ↑ Ovulation spurred by newfound semen ingredient
- ↑ Tuszynski MH, Blesch A (2004). "Nerve growth factor: from animal models of cholinergic neuronal degeneration to gene therapy in Alzheimer's disease". Prog. Brain Res. 146: 441–9. doi:10.1016/s0079-6123(03)46028-7. PMID 14699979.
- ↑ Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H et al. (September 2009). "The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model". Biomaterials 30 (27): 4649–56. doi:10.1016/j.biomaterials.2009.05.037. PMID 19573907.
- ↑ Freund V, Frossard N (2004). "Expression of nerve growth factor in the airways and its possible role in asthma". Prog. Brain Res. 146: 335–46. doi:10.1016/S0079-6123(03)46021-4. PMID 14712791.
- ↑ Althaus HH (2004). "Remyelination in multiple sclerosis: a new role for neurotrophins?". Prog. Brain Res. 146: 415–32. doi:10.1016/S0079-6123(03)46026-3. PMID 14699977.
- ↑ Villoslada P, Genain CP (2004). "Role of nerve growth factor and other trophic factors in brain inflammation". Prog. Brain Res. 146: 403–14. doi:10.1016/S0079-6123(03)46025-1. PMID 14699976.
- ↑ Chaldakov GN, Tonchev AB, Aloe L (2009). "NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines". Riv Psichiatr 44 (2): 79–87. PMID 20066808.
- ↑ Counts SE, Mufson EJ (April 2005). "The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease". J. Neuropathol. Exp. Neurol. 64 (4): 263–72. PMID 15835262.
- ↑ Hempstead BL (February 2006). "Dissecting the diverse actions of pro- and mature neurotrophins". Curr Alzheimer Res 3 (1): 19–24. doi:10.2174/156720506775697061. PMID 16472198.
- ↑ Allen SJ, Dawbarn D (February 2006). "Clinical relevance of the neurotrophins and their receptors". Clin. Sci. 110 (2): 175–91. doi:10.1042/CS20050161. PMID 16411894.
- ↑ Barry SR (December 1991). "Clinical implications of basic neuroscience research. II: NMDA receptors and neurotrophic factors". Arch Phys Med Rehabil 72 (13): 1095–101. PMID 1660257.
- ↑ Hefti F, Schneider LS (1991). "Nerve growth factor and Alzheimer's disease". Clin Neuropharmacol. 14 Suppl 1: S62–76. PMID 1913710.
- ↑ Olson L (1993). "NGF and the treatment of Alzheimer's disease". Exp. Neurol. 124 (1): 5–15. doi:10.1006/exnr.1993.1167. PMID 8282080.
- ↑ Parikh V, Evans DR, Khan MM, Mahadik SP (2003). "Nerve growth factor in never-medicated first-episode psychotic and medicated chronic schizophrenic patients: possible implications for treatment outcome". Schizophr. Res. 60 (2-3): 117–23. doi:10.1016/S0920-9964(02)00434-6. PMID 12591576.
- ↑ Lukoyanov NV, Pereira PA, Paula-Barbosa MM, Cadete-Leite A (1 January 2003). "Nerve growth factor improves spatial learning and restores hippocampal cholinergic fibers in rats withdrawn from chronic treatment with ethanol". Exp Brain Res 148 (1): 88–94. doi:10.1007/s00221-002-1290-7. PMID 12478399.
- ↑ Riikonen R, Vanhala R (1999). "Levels of cerebrospinal fluid nerve-growth factor differ in infantile autism and Rett syndrome". Dev Med Child Neurol 41 (3): 148–52. doi:10.1111/j.1469-8749.1999.tb00573.x. PMID 10210246.
- ↑ 35.0 35.1 Gorbachevskaya N, Bashina V, Gratchev V, Iznak A (December 2001). "Cerebrolysin therapy in Rett syndrome: clinical and EEG mapping study". Brain Dev. 23 Suppl 1: S90–3. doi:10.1016/S0387-7604(01)00349-7. PMID 11738849.
- ↑ Barbosa IG, Huguet RB, Neves FS, Reis HJ, Bauer ME, Janka Z et al. (2011). "Impaired nerve growth factor homeostasis in patients with bipolar disorder". World J. Biol. Psychiatry 12 (3): 228–32. doi:10.3109/15622975.2010.518629. PMID 20923384.
- ↑ Machado-Vieira R, Manji HK, Zarate CA (2009). "The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis". Bipolar Disord. 11 Suppl 2: 92–109. doi:10.1111/j.1399-5618.2009.00714.x. PMC 2800957. PMID 19538689.
- ↑ Scott SA, Mufson EJ, Weingartner JA, Skau KA, Crutcher KA (1995). "Nerve growth factor in Alzheimer's disease: increased levels throughout the brain coupled with declines in nucleus basalis". J. Neurosci. 15 (9): 6213–21. PMID 7666203.
- ↑ Chaldakov GN, Fiore M, Stankulov IS, Manni L, Hristova MG, Antonelli A et al. (2004). "Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: a role for NGF and BDNF in cardiovascular disease?". Prog. Brain Res. 146: 279–89. doi:10.1016/S0079-6123(03)46018-4. PMID 14699970.
- ↑ Chaldakov GN, Fiore M, Tonchev AB, Dimitrov D, Pancheva R, Rancic G et al. (2007). "Homo obesus: a metabotrophin-deficient species. Pharmacology and nutrition insight". Curr. Pharm. Des. 13 (21): 2176–9. doi:10.2174/138161207781039616. PMID 17627549.
- ↑ Manni L, Nikolova V, Vyagova D, Chaldakov GN, Aloe L (June 2005). "Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes". Int. J. Cardiol. 102 (1): 169–71. doi:10.1016/j.ijcard.2004.10.041. PMID 15939120.
- ↑ Kawamoto K, Matsuda H (2004). "Nerve growth factor and wound healing". Prog. Brain Res. 146: 369–84. doi:10.1016/S0079-6123(03)46023-8. PMID 14699974.
- ↑ Giudice LC (June 2010). "Clinical practice. Endometriosis". N. Engl. J. Med. 362 (25): 2389–98. doi:10.1056/NEJMcp1000274. PMC 3108065. PMID 20573927.
- ↑ Emanuele E, Politi P, Bianchi M, Minoretti P, Bertona M, Geroldi D (April 2006). "Raised plasma nerve growth factor levels associated with early-stage romantic love". Psychoneuroendocrinology 31 (3): 288–94. doi:10.1016/j.psyneuen.2005.09.002. PMID 16289361.
- ↑ "Molecule Gives Passionate Lovers Just One Year". Health News. redOrbit. 2005-11-25. Retrieved 2012-03-02.
- ↑ Harris J (2005-11-29). "The question: Is love just a chemical?". Education. Guardian. Retrieved 2012-03-02.
- ↑ 47.0 47.1 Popham P (2009-04-25). "Is this the secret of eternal life? - Science - News - The Independent". The Independent. Retrieved 2012-03-02.
- ↑ Balasubramanian S, Mintzer JE, Wahlquist AE (August 2014). "Induction of salivary nerve growth factor by Yogic breathing: a randomized controlled trial". Int Psychogeriatr 27 (1): 168–70. doi:10.1017/S1041610214001616. PMID 25101659. Check date values in:
|year= / |date= mismatch(help) - ↑ 49.0 49.1 Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS et al. (February 2004). "Sortilin is essential for proNGF-induced neuronal cell death". Nature 427 (6977): 843–8. doi:10.1038/nature02319. PMID 14985763.
- ↑ Wiesmann C, Ultsch MH, Bass SH, de Vos AM (September 1999). "Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor". Nature 401 (6749): 184–8. doi:10.1038/43705. PMID 10490030.
External links
- Nerve Growth Factor at the US National Library of Medicine Medical Subject Headings (MeSH)
- NGF for corneal therapeutic purposes
- NGF - twenty years a-growing
| |||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||