Nematode
| Roundworms Temporal range: Lower Cambrian – Recent,[1] | |
|---|---|
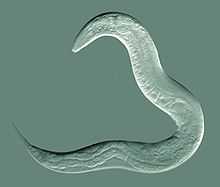 | |
| Caenorhabditis elegans, a model species of roundworm | |
| Scientific classification | |
| Kingdom: | Animalia |
| Clade: | Nematoida |
| Phylum: | Nematoda Diesing, 1861 |
| Classes | |
|
Chromadorea (disputed) | |
| Synonyms | |
|
Adenophorea (see text) | |
The nematodes /ˈnɛmətoʊdz/ or roundworms constitute the phylum Nematoda. They are a diverse animal phylum inhabiting a very broad range of environments. Nematode species can be difficult to distinguish, and although over 25,000 have been described,[2][3] of which more than half are parasitic, the total number of nematode species has been estimated to be about 1 million.[4] Unlike cnidarians and flatworms, nematodes have tubular digestive systems with openings at both ends.
Habitats
Nematodes have successfully adapted to nearly every ecosystem from marine (salt water) to fresh water, to soils, and from the polar regions to the tropics, as well as the highest to the lowest of elevations. They are ubiquitous in freshwater, marine, and terrestrial environments, where they often outnumber other animals in both individual and species counts, and are found in locations as diverse as mountains, deserts and oceanic trenches. They are found in every part of the earth's lithosphere.[5] They represent, for example, 90% of all life forms on the ocean floor.[6] Nematodes have even been found at great depth (0.9–3.6 km) below the surface of the Earth in gold mines in South Africa.[7][8][9][10][11] Their numerical dominance, often exceeding a million individuals per square meter and accounting for about 80% of all individual animals on earth, their diversity of life cycles, and their presence at various trophic levels point at an important role in many ecosystems.[12]
Their many parasitic forms include pathogens in most plants and animals (including humans).[13] Some nematodes can undergo cryptobiosis. One group of carnivorous fungi, the nematophagous fungi, are predators of soil nematodes. They set enticements for the nematodes in the form of lassos or adhesive structures.[14][15][16]
Nathan Cobb described the ubiquity of nematodes on Earth thus:
In short, if all the matter in the universe except the nematodes were swept away, our world would still be dimly recognizable, and if, as disembodied spirits, we could then investigate it, we should find its mountains, hills, vales, rivers, lakes, and oceans represented by a film of nematodes. The location of towns would be decipherable, since for every massing of human beings there would be a corresponding massing of certain nematodes. Trees would still stand in ghostly rows representing our streets and highways. The location of the various plants and animals would still be decipherable, and, had we sufficient knowledge, in many cases even their species could be determined by an examination of their erstwhile nematode parasites."[17]
Taxonomy and systematics
The group was originally defined by Karl Rudolphi in 1808[18] under the name Nematoidea, from Ancient Greek νῆμα (nêma, nêmatos, 'thread') and -eiδἠς (-eidēs, 'species'). It was reclassified as family Nematodes by Burmeister in 1837[18] and order Nematoda by K. M. Diesing in 1861.[18]
At its origin, the "Nematoidea" included both roundworms and horsehair worms. Along with Acanthocephala, Trematoda and Cestoidea, it formed the group Entozoa.[19] The first differentiation of roundworms from horsehair worms, though erroneous, is due to von Siebold (1843) with orders Nematoidea and Gordiacei (Gordiacea). They were classed along with Acanthocephala in the new phylum Nemathelminthes (today obsolete) by Gegenbaur (1859). The taxon Nematoidea, including the family Gordiidae (horsehair worms), was then promoted to the rank of phylum by Ray Lankester (1877). In 1919, Nathan Cobb proposed that roundworms should be recognized alone as a phylum. He argued they should be called nema(s) in English rather than "nematodes"[lower-alpha 1] and defined the taxon Nemates (Latin plural of nema). Since Cobb was the first to exclude all but nematodes from the group, some sources consider the valid taxon name to be Nemates or Nemata, rather than Nematoda.[20]
Phylogeny
The relationships of the nematodes and their close relatives among the protostomian Metazoa are unresolved. Traditionally, they were held to be a lineage of their own but in the 1990s, they were proposed to form the group Ecdysozoa together with moulting animals, such as arthropods. The identity of the closest living relatives of the Nematoda has always been considered to be well resolved. Morphological characters and molecular phylogenies agree with placement of the roundworms as a sister taxon to the parasitic horsehair worms (Nematomorpha); together they make up the Nematoida. Together with the Scalidophora (formerly Cephalorhyncha), the Nematoida form the Introverta. It is entirely unclear whether the Introverta are, in turn, the closest living relatives of the enigmatic Gastrotricha; if so, they are considered a clade Cycloneuralia, but much disagreement occurs both between and among the available morphological and molecular data. The Cycloneuralia or the Introverta—depending on the validity of the former—are often ranked as a superphylum.[21]
Nematode systematics
Due to the lack of knowledge regarding many nematodes, their systematics is contentious. An earliest and influential classification was proposed by Chitwood and Chitwood[22]—later revised by Chitwood[23]—who divided the phylum into two—the Aphasmidia and the Phasmidia. These were later renamed Adenophorea (gland bearers) and Secernentea (secretors), respectively.[24] The Secernentea share several characteristics, including the presence of phasmids, a pair of sensory organs located in the lateral posterior region, and this was used as the basis for this division. This scheme was adhered to in many later classifications, though the Adenophorea were not a uniform group.
Initial DNA sequence studies suggested the existence of five clades:[25]
- Dorylaimia
- Enoplia
- Spirurina
- Tylenchina
- Rhabditina
As it seems, the Secernentea are indeed a natural group of closest relatives. But the "Adenophorea" appear to be a paraphyletic assemblage of roundworms simply retaining a good number of ancestral traits. The old Enoplia do not seem to be monophyletic either, but to contain two distinct lineages. The old group "Chromadoria" seem to be another paraphyletic assemblage, with the Monhysterida representing a very ancient minor group of nematodes. Among the Secernentea, the Diplogasteria may need to be united with the Rhabditia, while the Tylenchia might be paraphyletic with the Rhabditia.[26]
The understanding of roundworm systematics and phylogeny as of 2002 is summarised below:
Phylum Nematoda
- Basal order Monhysterida
- Class Dorylaimea
- Class Enoplea
- Class Secernentea
- "Chromadorea" assemblage
Later work has suggested the presence of 12 clades.[27] The Secernentea—a group that includes virtually all major animal and plant 'nematode' parasites—apparently arose from within the Adenophorea.
A major effort to improve the systematics of this phylum is in progress and being organised by the 959 Nematode Genomes.[28]
A complete checklist of the World's nematode species can be found in the World Species Index:Nematoda.[29]
An analysis of the mitochondrial DNA suggests that the following groupings are valid[30]
- subclass Dorylaimia
- orders Rhabditida, Trichinellida and Mermithida
- suborder Rhabditina
- infraorders Spiruromorpha and Oxyuridomorpha
The Ascaridomorpha, Rhabditomorpha and Diplogasteromorpha appear to be related.
The suborders Spirurina and Tylenchina and the infraorders Rhabditomorpha, Panagrolaimomorpha and Tylenchomorpha are paraphytic.
The monophyly of the Ascaridomorph is uncertain.
Anatomy
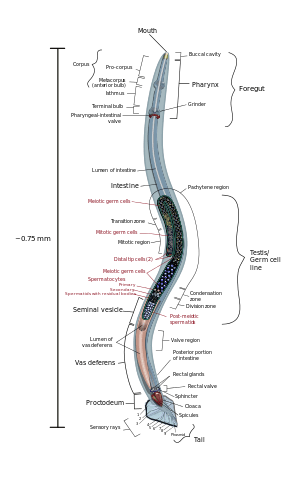
Nematodes are slender worms: typically approximately 5 to 100 µm thick, and at least 0.1 mm (0.0039 in) but less than 2.5mm long.[31] The smallest nematodes are microscopic, while free-living species can reach as much as 5 cm (2.0 in), and some parasitic species are larger still, reaching over a meter in length.[32]:271 The body is often ornamented with ridges, rings, bristles, or other distinctive structures.[33]
The head of a nematode is relatively distinct. Whereas the rest of the body is bilaterally symmetrical, the head is radially symmetrical, with sensory bristles and, in many cases, solid 'head-shields' radiating outwards around the mouth. The mouth has either three or six lips, which often bear a series of teeth on their inner edges. An adhesive 'caudal gland' is often found at the tip of the tail.[34]
The epidermis is either a syncytium or a single layer of cells, and is covered by a thick collagenous cuticle. The cuticle is often of complex structure, and may have two or three distinct layers. Underneath the epidermis lies a layer of longitudinal muscle cells. The relatively rigid cuticle works with the muscles to create a hydroskeleton as nematodes lack circumferential muscles. Projections run from the inner surface of muscle cells towards the nerve cords; this is a unique arrangement in the animal kingdom, in which nerve cells normally extend fibres into the muscles rather than vice versa.[34]
Digestive system
The oral cavity is lined with cuticle, which is often strengthened with ridges or other structures, and, especially in carnivorous species, may bear a number of teeth. The mouth often includes a sharp stylet, which the animal can thrust into its prey. In some species, the stylet is hollow, and can be used to suck liquids from plants or animals.[34]
The oral cavity opens into a muscular, sucking pharynx, also lined with cuticle. Digestive glands are found in this region of the gut, producing enzymes that start to break down the food. In stylet-bearing species, these may even be injected into the prey.[34]
There is no stomach, with the pharynx connecting directly to a muscleless intestine that forms the main length of the gut. This produces further enzymes, and also absorbs nutrients through its single cell thick lining. The last portion of the intestine is lined by cuticle, forming a rectum, which expels waste through the anus just below and in front of the tip of the tail. Movement of food through the digestive system is the result of body movements of the worm. The intestine has valves or sphincters at either end to help control the movement of food through the body.[34]
Excretory system
Nitrogenous waste is excreted in the form of ammonia through the body wall, and is not associated with any specific organs. However, the structures for excreting salt to maintain osmoregulation are typically more complex.[34]
In many marine nematodes, one or two unicellular 'renette glands' excrete salt through a pore on the underside of the animal, close to the pharynx. In most other nematodes, these specialised cells have been replaced by an organ consisting of two parallel ducts connected by a single transverse duct. This transverse duct opens into a common canal that runs to the excretory pore.[34]
Nervous system
Four peripheral nerves run the length of the body on the dorsal, ventral, and lateral surfaces. Each nerve lies within a cord of connective tissue lying beneath the cuticle and between the muscle cells. The ventral nerve is the largest, and has a double structure forward of the excretory pore. The dorsal nerve is responsible for motor control, while the lateral nerves are sensory, and the ventral combines both functions.[34]
The nervous system is also the only place in the nematode body that contains cilia, which are all non-motile and with a sensory function.[35][36]
At the anterior end of the animal, the nerves branch from a dense, circular nerve ring surrounding the pharynx, and serving as the brain. Smaller nerves run forward from the ring to supply the sensory organs of the head.[34]
The bodies of nematodes are covered in numerous sensory bristles and papillae that together provide a sense of touch. Behind the sensory bristles on the head lie two small pits, or 'amphids'. These are well supplied with nerve cells, and are probably chemoreception organs. A few aquatic nematodes possess what appear to be pigmented eye-spots, but is unclear whether or not these are actually sensory in nature.[34]
Reproduction
Most nematode species are dioecious, with separate male and female individuals. Both sexes possess one or two tubular gonads. In males, the sperm are produced at the end of the gonad, and migrate along its length as they mature. The testes each open into a relatively wide sperm duct and then into a glandular and muscular ejaculatory duct associated with the cloaca. In females, the ovaries each open into an oviduct and then a glandular uterus. The uteri both open into a common vagina, usually located in the middle of the ventral surface.[34]
Reproduction is usually sexual. Males are usually smaller than females (often much smaller) and often have a characteristically bent tail for holding the female. During copulation, one or more chitinized spicules move out of the cloaca and are inserted into the genital pore of the female. Amoeboid sperm crawl along the spicule into the female worm. Nematode sperm is thought to be the only eukaryotic cell without the globular protein G-actin.
Eggs may be embryonated or unembryonated when passed by the female, meaning their fertilized eggs may not yet be developed. A few species are known to be ovoviviparous. The eggs are protected by an outer shell, secreted by the uterus. In free-living roundworms, the eggs hatch into larvae, which appear essentially identical to the adults, except for an underdeveloped reproductive system; in parasitic roundworms, the life cycle is often much more complicated.[34]
Nematodes as a whole possess a wide range of modes of reproduction.[38] Some nematodes, such as Heterorhabditis spp., undergo a process called endotokia matricida: intrauterine birth causing maternal death.[39] Some nematodes are hermaphroditic, and keep their self-fertilized eggs inside the uterus until they hatch. The juvenile nematodes will then ingest the parent nematode. This process is significantly promoted in environments with a low food supply.[39]
The nematode model species Caenorhabditis elegans and C. briggsae exhibit androdioecy, which is very rare among animals. The single genus Meloidogyne (root-knot nematodes) exhibit a range of reproductive modes, including sexual reproduction, facultative sexuality (in which most, but not all, generations reproduce asexually), and both meiotic and mitotic parthenogenesis.
The genus Mesorhabditis exhibits an unusual form of parthenogenesis, in which sperm-producing males copulate with females, but the sperm do not fuse with the ovum. Contact with the sperm is essential for the ovum to begin dividing, but because there is no fusion of the cells, the male contributes no genetic material to the offspring, which are essentially clones of the female.[34]
Free-living species
In free-living species, development usually consists of four molts of the cuticle during growth. Different species feed on materials as varied as algae, fungi, small animals, fecal matter, dead organisms and living tissues. Free-living marine nematodes are important and abundant members of the meiobenthos. They play an important role in the decomposition process, aid in recycling of nutrients in marine environments, and are sensitive to changes in the environment caused by pollution. One roundworm of note, Caenorhabditis elegans, lives in the soil and has found much use as a model organism. C. elegans has had its entire genome sequenced, as well as the developmental fate of every cell determined, and every neuron mapped.
Parasitic species

Nematodes commonly parasitic on humans include ascarids (Ascaris), filarias, hookworms, pinworms (Enterobius) and whipworms (Trichuris trichiura). The species Trichinella spiralis, commonly known as the 'trichina worm', occurs in rats, pigs, and humans, and is responsible for the disease trichinosis. Baylisascaris usually infests wild animals, but can be deadly to humans, as well. Dirofilaria immitis are known for causing heartworm disease by inhabiting the hearts, arteries, and lungs of dogs and some cats. Haemonchus contortus is one of the most abundant infectious agents in sheep around the world, causing great economic damage to sheep. In contrast, entomopathogenic nematodes parasitize insects and are mostly considered beneficial by humans, but some attack beneficial insects.
One form of nematode is entirely dependent upon fig wasps, which are the sole source of fig fertilization. They prey upon the wasps, riding them from the ripe fig of the wasp's birth to the fig flower of its death, where they kill the wasp, and their offspring await the birth of the next generation of wasps as the fig ripens.
A newly discovered parasitic tetradonematid nematode, Myrmeconema neotropicum, apparently induces fruit mimicry in the tropical ant Cephalotes atratus. Infected ants develop bright red gasters (abdomens), tend to be more sluggish, and walk with their gasters in a conspicuous elevated position. It is likely that these changes cause frugivorous birds to confuse the infected ants for berries, and eat them. Parasite eggs passed in the bird's feces are subsequently collected by foraging Cephalotes atratus and are fed to their larvae, thus completing the life cycle of M. neotropicum.[40]
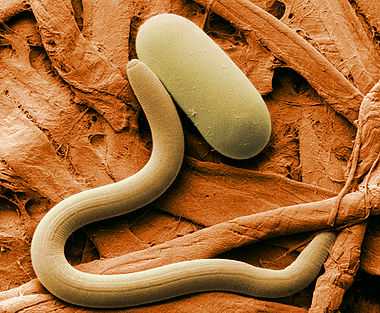
Plant-parasitic nematodes include several groups causing severe crop losses. The most common genera are Aphelenchoides (foliar nematodes), Ditylenchus, Globodera (potato cyst nematodes), Heterodera (soybean cyst nematodes), Longidorus, Meloidogyne (root-knot nematodes), Nacobbus, Pratylenchus (lesion nematodes), Trichodorus and Xiphinema (dagger nematodes). Several phytoparasitic nematode species cause histological damages to roots, including the formation of visible galls (e.g. by root-knot nematodes), which are useful characters for their diagnostic in the field. Some nematode species transmit plant viruses through their feeding activity on roots. One of them is Xiphinema index, vector of grapevine fanleaf virus, an important disease of grapes, another one is Xiphinema diversicaudatum, vector of arabis mosaic virus.
Other nematodes attack bark and forest trees. The most important representative of this group is Bursaphelenchus xylophilus, the pine wood nematode, present in Asia and America and recently discovered in Europe.
Agriculture and horticulture
Depending on the species, a nematode may be beneficial or detrimental to plant health. From agricultural and horticulture perspectives, the two categories of nematodes are the predatory ones, which will kill garden pests like cutworms and corn earworm moths, and the pest nematodes, like the root-knot nematode, which attack plants, and those that act as vectors spreading plant viruses between crop plants.[41] Predatory nematodes can be bred by soaking a specific recipe of leaves and other detritus in water, in a dark, cool place, and can even be purchased as an organic form of pest control.
Rotations of plants with nematode-resistant species or varieties is one means of managing parasitic nematode infestations. For example, marigolds, grown over one or more seasons (the effect is cumulative), can be used to control nematodes.[42] Another is treatment with natural antagonists such as the fungus Gliocladium roseum. Chitosan, a natural biocontrol, elicits plant defense responses to destroy parasitic cyst nematodes on roots of soybean, corn, sugar beet, potato and tomato crops without harming beneficial nematodes in the soil.[43] Soil steaming is an efficient method to kill nematodes before planting a crop, but indiscriminately eliminates both harmful and beneficial soil fauna.
The Golden Nematode (Globodera rostochiensis) is a particularly harmful variety of nematode pest that has resulted in quarantines and crop failures worldwide. CSIRO has found[44] a 13- to 14-fold reduction of nematode population densities in plots having Indian mustard (Brassica juncea) green manure or seed meal in the soil.
Hundreds of Caenorhabditis elegans were featured in a research project on NASA's STS-107 space mission, and were known to have survived the Space Shuttle Columbia disaster.[45]
Epidemiology

A number of intestinal nematodes cause diseases affecting human beings, including ascariasis, trichuriasis and hookworm disease. Filarial nematodes cause filariasis.
Soil ecosystems
90 percent of nematodes reside in the top 15 cm of soil. Nematodes do not decompose organic matter, but, instead, are parasitic and free-living organisms that feed on living material. Nematodes can effectively regulate bacterial population and community composition - they may eat up to 5,000 bacteria per minute. Also, Nematodes can play an important role in the nitrogen cycle by way of nitrogen mineralization.[31]
See also
- Biological pest control
- Capillaria
- Caenorhabditis elegans: An important model organism often used to study cellular differentiation, sometimes simply referred to as "worm" by scientists
- List of organic gardening and farming topics
- List of parasites of humans
- Toxocariasis: A helminth infection of humans caused by the dog or cat roundworm, Toxocara canis or Toxocara cati
Notes
- ↑ Note that words as nematologist, nematosis, nematocide, etc. are based on nema, nematos and not on "nematode".
References
- ↑ "Nematode Fossils." Nematode Fossils [Nematoda]. N.p., n.d. Web. 21 Apr. 2013.
- ↑ Hodda, M (2011). "Phylum Nematoda Cobb, 1932. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness". Zootaxa 3148: 63–95.
- ↑ Zhang, Z (2013). "Animal biodiversity: An update of classification and diversity in 2013. In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013)". Zootaxa 3703 (1): 5–11. doi:10.11646/zootaxa.3703.1.3.
- ↑ Lambshead PJD (1993). "Recent developments in marine benthic biodiversity research". Oceanis 19 (6): 5–24.
- ↑ Borgonie G, García-Moyano A, Litthauer D, Bert W, Bester A, van Heerden E, Möller C, Erasmus M, Onstott TC (June 2011). "Nematoda from the terrestrial deep subsurface of South Africa". Nature 474 (7349): 79–82. doi:10.1038/nature09974. PMID 21637257.
- ↑ Danovaro R, Gambi C, Dell'Anno A, Corinaldesi C, Fraschetti S, Vanreusel A, Vincx M, Gooday AJ (January 2008). "Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss". Curr. Biol. 18 (1): 1–8. doi:10.1016/j.cub.2007.11.056. PMID 18164201. Lay summary – EurekAlert!.
- ↑ Lemonick MD (2011-06-08). "Could 'worms from Hell' mean there's life in space?". Time. ISSN 0040-781X. Retrieved 2011-06-08.
- ↑ Bhanoo SN (2011-06-01). "Nematode found in mine is first subsurface multicellular organism". The New York Times. ISSN 0362-4331. Retrieved 2011-06-13.
- ↑ "Gold mine". Nature 474 (7349): 6. June 2011. doi:10.1038/474006b.
- ↑ Drake N (2011-06-01). "Subterranean worms from hell: Nature News". Nature News. Retrieved 2011-06-13.
- ↑ Borgonie G, García-Moyano A, Litthauer D, Bert W, Bester A, van Heerden E, Möller C, Erasmus M, Onstott TC (2011-06-02). "Nematoda from the terrestrial deep subsurface of South Africa". Nature 474 (7349): 79–82. doi:10.1038/nature09974. ISSN 0028-0836. PMID 21637257.
- ↑ Platt HM (1994). "foreword". In Lorenzen S, Lorenzen SA. The phylogenetic systematics of freeliving nematodes. London: The Ray Society. ISBN 0-903874-22-9.
- ↑ Hsueh YP, Leighton DHW, Sternberg PW. (2014). Nematode Communication. In: Witzany G (ed). Biocommunication of Animals. Springer, 383-407. ISBN 978-94-007-7413-1.
- ↑ Pramer C (1964). "Nematode-trapping fungi". Science 144 (3617): 382–388. doi:10.1126/science.144.3617.382. PMID 14169325.
- ↑ Hauser JT (December 1985). "Nematode-trapping fungi" (PDF). Carnivorous Plant Newsletter 14 (1): 8–11.
- ↑ Ahrén D, Ursing BM, Tunlid A (1998). "Phylogeny of nematode-trapping fungi based on 18S rDNA sequences". FEMS Microbiology Letters 158 (2): 179–184. doi:10.1016/s0378-1097(97)00519-3. PMID 9465391.
- ↑ Cobb, Nathan (1914). "Nematodes and their relationships". Yearbook United States Department of Agriculture. United States Department of Agriculture. pp. 457–90. Quote on p. 472.
- ↑ 18.0 18.1 18.2 Chitwood BG (1957). "The English word "Nema" Revised". Systematic Zoology in Nematology Newsletter 4 (45): 1619. doi:10.2307/sysbio/6.4.184.
- ↑ Siddiqi MR (2000). Tylenchida: parasites of plants and insects. Wallingford, Oxon, UK: CABI Pub. ISBN 0-85199-202-1.
- ↑ "ITIS report: Nematoda". Itis.gov. Retrieved 2012-06-12.
- ↑ "Bilateria". Tree of Life Web Project (ToL). January 1, 2002. Retrieved 2008-11-02.
- ↑ Chitwood BG, Chitwood MB (1933). "The characters of a protonematode". J Parasitol 20: 130.
- ↑ Chitwood BG (1937). "A revised classification of the Nematoda". Papers on helminthology, 30 year jubileum K.J. Skrjabin. Moscow: All-Union Lenin Academy of Agricultural Sciences. pp. 67–79.
- ↑ Chitwood BG (1958). "The designation of official names for higher taxa of invertebrates". Bull Zool Nomencl 15: 860–95.
- ↑ Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT, Thomas WK (March 1998). "A molecular evolutionary framework for the phylum Nematoda". Nature 392 (6671): 71–5. doi:10.1038/32160. PMID 9510248.
- ↑ "Nematoda". Tree of Life Web Project (ToL). 2002-01-01. Retrieved 2008-11-02.
- ↑ Holterman M, van der Wurff A, van den Elsen S, van Megen H, Bongers T, Holovachov O, Bakker J, Helder J (2006). "Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown Clades". Mol Biol Evol 23 (9): 1792–1800. doi:10.1093/molbev/msl044. PMID 16790472.
- ↑ "959 Nematode Genomes – NematodeGenomes". Nematodes.org. 2011-11-11. Retrieved 2012-06-12.
- ↑ World Species Index:Nematoda. 2012.
- ↑ Liu, GH; Shao, R; Li, JY; Zhou, DH; Li, H; Zhu, XQ (2013). "The complete mitochondrial genomes of three parasitic nematodes of birds: a unique gene order and insights into nematode phylogeny". BMC Genomics 14 (1): 414. doi:10.1186/1471-2164-14-414.
- ↑ 31.0 31.1 Nyle C. Brady & Ray R. Weil (2009). Elements of the Nature and Properties of Soils (3rd Edition). Prentice Hall. ISBN 9780135014332.
- ↑ Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology: A Functional Evolutionary Approach (7th ed.). Belmont, California: Brooks/Cole. ISBN 978-0-03-025982-1.
- ↑ Weischer B, Brown DJF (2000). An Introduction to Nematodes: General Nematology. Sofia, Bulgaria: Pensoft. pp. 75–76. ISBN 978-954-642-087-9.
- ↑ 34.0 34.1 34.2 34.3 34.4 34.5 34.6 34.7 34.8 34.9 34.10 34.11 34.12 Barnes RG (1980). Invertebrate zoology. Philadelphia: Sanders College. ISBN 0-03-056747-5.
- ↑ The sensory cilia of Caenorhabditis elegans
- ↑ Hearing in Drosophila Requires TilB, a Conserved Protein Associated With Ciliary Motility
- ↑ Lalošević, V.; Lalošević, D.; Capo, I.; Simin, V.; Galfi, A.; Traversa, D. (2013). "High infection rate of zoonotic Eucoleus aerophilus infection in foxes from Serbia.". Parasite 20: 3. doi:10.1051/parasite/2012003. PMC 3718516. PMID 23340229.
- ↑ Bell G (1982). The masterpiece of nature: the evolution and genetics of sexuality. Berkeley: University of California Press. ISBN 0-520-04583-1.
- ↑ 39.0 39.1 Johnigk S-A, Ehlers R-U (1999). "Endotokia matricida in hermaphrodites of Heterorhabditis spp. and the effect of the food supply". Nematology 1 (7–8): 717–726. doi:10.1163/156854199508748. ISSN 1388-5545.
- ↑ Yanoviak SP, Kaspari M, Dudley R, Poinar G (April 2008). "Parasite-induced fruit mimicry in a tropical canopy ant". Am. Nat. 171 (4): 536–44. doi:10.1086/528968. PMID 18279076.
- ↑ Purcell, M., M.W. Johnson, L.M. Lebeck, and A.H. Hara. 1992. Biological control of Helicoverpa zea (Lepidoptera: Noctuidae) with Steinernema carpocapsae (Rhabditida: Steinernematidae) in corn used as a trap crop. Environmental Entomology 21:1441-1447.
- ↑ Riotte L (1975). Secrets of companion planting for successful gardening. p. 7.
- ↑ US application 2008072494, Stoner RJ, Linden JC, "Micronutrient elicitor for treating nematodes in field crops", published 2008-03-27
- ↑ "CSIRO Publishing – Australasian plant pathology". www.publish.csiro.au. Retrieved 2010-06-14.
- ↑ "Worms survived Columbia disaster". Science/Nature. BBC News. 2003-04-01.
Further reading
- Atkinson, H.J. (1973). "The respiratory physiology of the marine nematodes Enoplus brevis (Bastian) and E. communis (Bastian): I. The influence of oxygen tension and body size" (PDF). J. Exp. Biol. 59 (1): 255–266.
- BBC News (2003): Worms survived Columbia disaster. Version of 2003-May-01. Retrieved 2008-Nov-04.
- Gubanov, N.M. (1951). "Giant nematoda from the placenta of Cetacea; Placentonema gigantissima nov. gen., nov. sp.".". Proc. USSR Acad. Sci. 77 (6): 1123–1125. [in Russian].
- Kaya, Harry K. et al (1993). "An Overview of Insect-Parasitic and Entomopathogenic Nematodes". In Bedding, R.A. Nematodes and the Biological Control of Insect Pests. Csiro Publishing. ISBN 9780643105911.
- Merck Veterinary Manual (MVM) (2006): Giant kidney worm infection in mink and dogs. Retrieved 2007-FEB-10.
- White JG, Southgate E, Thomson JN, Brenner S (August 1976). "The structure of the ventral nerve cord of Caenorhabditis elegans". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 275 (938): 327–48. doi:10.1098/rstb.1976.0086. PMID 8806.
- Lee, Donald L, ed. (2010). The biology of nematodes. London: Taylor & Francis. ISBN 0415272114. Retrieved 16 December 2014.
- De Ley, P & Blaxter, M 2004, 'A new system for Nematoda: combining morphological characters with molecular trees, and translating clades into ranks and taxa'. in R Cook & DJ Hunt (eds), Nematology Monographs and Perspectives. vol. 2, E.J. Brill, Leiden, pp. 633–653.
External links
- Harper Adams University College Nematology Research
- Nematodes/roundworms of man
- http://www.ucmp.berkeley.edu/phyla/ecdysozoa/nematoda.html
- European Society of Nematologists
- Nematode.net: Repository of parasitic nematode sequences.
- http://www.nematodes.org/
- NeMys World free-living Marine Nematodes database
- Nematode Virtual Library
- International Federation of Nematology Societies
- Society of Nematologists
- Australasian Association of Nematologists
- Research on nematodes and longevity
- Nematode on BBC
- Nematode worms in an aquarium
- Phylum Nematoda – nematodes on the UF / *IFAS Featured Creatures Web site
| Wikimedia Commons has media related to Nematoda. |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
