NCS-382
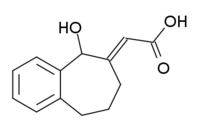 | |
| Names | |
|---|---|
| IUPAC name
(2E)-(5-hydroxy-5,7,8,9-tetrahydro-6H-benzo[a][7]annulen-6-ylidene ethanoic acid | |
| Identifiers | |
| 131733-92-1 | |
| ChemSpider | 4730868 |
| |
| Jmol-3D images | Image |
| MeSH | NCS-382 |
| PubChem | 5875451 |
| |
| Properties | |
| C13H14O3 | |
| Molar mass | 218.248 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
NCS-382 is a moderately selective antagonist for the GHB receptor.[1][2] It blocks the effects of GHB in animals and has both anti-sedative and anticonvulsant effects.[3][4][5] It has been proposed as a treatment for GHB overdose in humans, as well as for the endogenous metabolic disorder succinic semialdehyde dehydrogenase deficiency, but has never been developed for clinical use.[6]
References
- ↑ Castelli MP, Pibiri F, Carboni G, Piras AP (2004). "A review of pharmacology of NCS-382, a putative antagonist of gamma-hydroxybutyric acid (GHB) receptor" (PDF). CNS Drug Reviews 10 (3): 243–260. doi:10.1111/j.1527-3458.2004.tb00025.x. PMID 15492774.
- ↑ Ticku MK, Mehta AK (October 2008). "Characterization and pharmacology of the GHB receptor". Annals of the New York Academy of Sciences 1139: 374–385. doi:10.1196/annals.1432.048. PMID 18991884.
- ↑ Maitre M, Hechler V, Vayer P, Gobaille S, Cash CD, Schmitt M, Bourguignon JJ (Nov 1990). "A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties". Journal of Pharmacology and Experimental Therapeutics 255 (2): 657–563. PMID 2173754.
- ↑ Schmidt C, Gobaille S, Hechler V, Schmitt M, Bourguignon JJ, Maitre M (Oct 1991). "Anti-sedative and anti-cataleptic properties of NCS-382, a gamma-hydroxybutyrate receptor antagonist". European Journal of Pharmacology 203 (3): 393–297. doi:10.1016/0014-2999(91)90896-X. PMID 1773824.
- ↑ Colombo G, Agabio R, Bourguignon J, Fadda F, Lobina C, Maitre M, Reali R, Schmitt M, Gessa GL (Sep 1995). "Blockade of the discriminative stimulus effects of gamma-hydroxybutyric acid (GHB) by the GHB receptor antagonist NCS-382". Physiology and Behaviour 58 (3): 587–590. doi:10.1016/0031-9384(95)00086-X. PMID 8587968.
- ↑ Gupta M, Greven R, Jansen EE, Jakobs C, Hogema BM, Froestl W, Snead OC, Bartels H, Grompe M, Gibson KM (Jul 2002). "Therapeutic intervention in mice deficient for succinate semialdehyde dehydrogenase (gamma-hydroxybutyric aciduria)" (PDF). Journal of Pharmacology and Experimental Therapeutics 302 (1): 180–187. doi:10.1124/jpet.302.1.180. PMID 12065715.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||