N,N'-diacetylchitobiose phosphorylase
| N,N'-diacetylchitobiose phosphorylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.4.1.280 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
N,N'-diacetylchitobiose phosphorylase (EC 2.4.1.280, chbP (gene)) is an enzyme with the system name N,N'-diacetylchitobiose:phosphate N-acetyl-D-glucosaminyltransferase.[1][2][3] This enzyme was found in the genus Vibrio initially but has now been found to be taken up by Escherichia coli as well as many other bacteria. One study shows that Escherichia coli can replicate on a medium that is just composed of GlcNAc a product of phosphorylation of N,N'-diacetylchitobiose as the sole source of carbon. Because E. coli can go on this medium, the enzyme is present. The enzyme has also been found in multiple eukaryotic cells as well, especially in eukaryotes that make chitin and break chitin down. It is believed that N,N'-diacetylchitobiose phosphorylase is an integral part of the phosphoenolpyruvate:glucose phosphotransferase system (PTS). It is assumed that it is involved with Enzyme Complex II of the PTS and is involved with the synthesis of chitin.[4] The enzyme is specific for N,N'-diacetylchitobiose.
Mechanism
This enzyme catalyses the following chemical reaction
- N,N'-diacetylchitobiose + phosphate
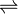 N-acetyl-D-glucosamine + N-acetyl-alpha-D-glucosamine 1-phosphate
N-acetyl-D-glucosamine + N-acetyl-alpha-D-glucosamine 1-phosphate
The mechanism for this reaction is as follows the enzymatic phosphorolysis of N,N’-diacetylchitobiose phosphorylase begins with the direct nucleophilic attack to the glycosidic bond with the aid of D492 or aspartic acid residue 492 of the active site of the enzyme, which donates a proton to the glycosidic oxygen atom, and then proceeds through an oxocarbenium cation activated by ion-like transition state.[1]
References
- ↑ 1.0 1.1 Park, J.K., Keyhani, N.O. and Roseman, S. (2000). "Chitin catabolism in the marine bacterium Vibrio furnissii. Identification, molecular cloning, and characterization of a N,N′-diacetylchitobiose phosphorylase". J. Biol. Chem. 275: 33077–33083. doi:10.1074/jbc.m001042200''. PMID 10913116.
- ↑ Honda, Y., Kitaoka, M. and Hayashi, K. (2004). "Reaction mechanism of chitobiose phosphorylase from Vibrio proteolyticus: identification of family 36 glycosyltransferase in Vibrio". Biochem. J. 377: 225–232. doi:10.1042/bj20031171. PMID 13678418.
- ↑ Hidaka, M., Honda, Y., Kitaoka, M., Nirasawa, S., Hayashi, K., Wakagi, T., Shoun, H. and Fushinobu, S. (2004). "Chitobiose phosphorylase from Vibrio proteolyticus', a member of glycosyl transferase family 36, has a clan GH-L-like (α/α)6 barrel fold". Structure 12: 937–947. doi:10.1016/j.str.2004.03.027. PMID 15274915.
- ↑ Keyhani, N.O., Wang, L., Lee, Y.C. and Roseman, S. (2000). "The Chitin Disaccharide, N,N'-Diacetylchitobiose, Is Catabolized by Escherichia coli and Is Transported/Phosphorylated by the Phosphoenolpyruvate:Glycose Phosphotransferase System". J. Biol. Chem. 275: 33084–33090. doi:10.1074/jbc.M001043200.
External links
- N,N'-diacetylchitobiose phosphorylase at the US National Library of Medicine Medical Subject Headings (MeSH)