Montmorillonite
| Montmorillonite | |
|---|---|
|
A sample of montmorillonite (unknown scale) | |
| General | |
| Category |
Phyllosilicates Smectite group |
| Formula (repeating unit) | (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O |
| Crystal symmetry |
Monoclinic prismatic H-M symbol: 2/m Space group: C2/m |
| Unit cell | a = 5.17 Å, b = 8.94 Å, c = 9.95 Å; β = 99.54°; Z = 1 |
| Identification | |
| Color | White, pale pink, blue, yellow, red, green |
| Crystal habit | compact masses of lamellar or globular microcrystalline aggregates |
| Crystal system | Monoclinic |
| Cleavage | {001} perfect |
| Fracture | Uneven |
| Mohs scale hardness | 1–2 |
| Luster | Dull, earthy |
| Diaphaneity | Translucent |
| Specific gravity | 1.7-2 |
| Optical properties | Biaxial (-) |
| Refractive index | nα = 1.485–1.535 nβ = 1.504–1.550 nγ = 1.505–1.550 |
| Birefringence | δ = 0.020 |
| 2V angle | Measured: 5° to 30° |
| References | [1][2][3] |
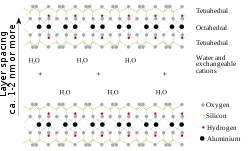
Montmorillonite is a very soft phyllosilicate group of minerals that typically form in microscopic crystals, forming a clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite group, is a 2:1 clay, meaning that it has 2 tetrahedral sheets sandwiching a central octahedral sheet. The particles are plate-shaped with an average diameter of approximately one micrometre. Members of this group include saponite.
Montmorillonite is a subclass of smectite, a 2:1 phyllosilicate mineral being characterized as having greater than 50% octahedral charge; its cation exchange capacity is due to isomorphous substitution of Mg for Al in the gibbsitic plane. In contrast, beidellite is smectite with greater than 50% tetrahedral charge originating from isomorphous substitution of Al for Si in the quartz sheet.
The water content of montmorillonite is variable and it increases greatly in volume when it absorbs water. Chemically it is hydrated sodium calcium aluminium magnesium silicate hydroxide (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O. Potassium, iron, and other cations are common substitutes, the exact ratio of cations varies with source. It often occurs intermixed with chlorite, muscovite, illite, cookeite, and kaolinite.
Cave conditions
Montmorillonite can be concentrated and transformed within cave environments. The natural weathering of the cave can leave behind concentrations of aluminosilicates which were contained within the bedrock. Montmorillonite can form slowly in solutions of aluminosilicates. High HCO3 concentrations and long periods of time can aid in the formation. Montmorillonite can then transform to palygorskite under dry conditions and to halloysite-10Å (endellite) in acidic conditions (pH 5 or lower). Halloysite-10Å can further transform into halloysite-7Å by drying.[4]
Uses
Montmorillonite is used in the oil drilling industry as a component of drilling mud, making the mud slurry viscous which helps in keeping the drill bit cool and removing drilled solids. It is also used as a soil additive to hold soil water in drought prone soils, to the construction of earthen dams and levees and to prevent the leakage of fluids. It is also used as a component of foundry sand and as a desiccant to remove moisture from air and gases.
Montmorillonite clays have been extensively utilized in catalytic processes. Catalytic cracking catalysts have used montmorillonite clays for over 60 years. Other acid based catalysts also utilize acid treated montmorillonite clays.[5]
Similar to many other clays, montmorillonite swells with the addition of water. However, some montmorillonites expand considerably more than other clays due to water penetrating the interlayer molecular spaces and concomitant adsorption. The amount of expansion is due largely to the type of exchangeable cation contained in the sample. The presence of sodium as the predominant exchangeable cation can result in the clay swelling to several times its original volume. Hence, sodium montmorillonite has come to be used as the major constituent in non-explosive agents for splitting rock in natural stone quarries in order to limit the amount of waste, or for the demolition of concrete structures where the use of explosive charges is unacceptable.
This swelling property makes montmorillonite-containing bentonite useful also as an annular seal or plug for water wells and as a protective liner for landfills. Other uses include as an anti-caking agent in animal feed, in paper making to minimize deposit formation and as a retention and drainage aid component. Montmorillonite has also been used in cosmetics.
In a fine powder form, it can also be used as a flocculent in ponds. Tossed on the surface as it drops into the water, making the water "clouded", it attracts minute particles in the water and then settles to the bottom, cleaning the water. Koi and Goldfish (carp)then actually feed on the "clump" which can aid in the digestion of the fish. Sold in pond supply shops.
Sodium montmorillonite is also used as the base of some cat litter products, due to its adsorbent and clumping properties.
Calcined clay products
Montmorillonite can be calcined to produce arcillite, a porous, calcined clay sold as a soil conditioner for playing fields and other soil products such as for use as bonsai soil as an alternative to akadama.
Use in medicine and pharmacology
Montmorillonite is effective as an adsorptive of heavy metals.[6]
For external use, montmorillonite has been used to treat contact dermatitis.[7]
Discovery
Montmorillonite was first described in 1847 for an occurrence in Montmorillon in the department of Vienne, France,[2] more than 50 years before the discovery of bentonite in the US. It is found in many locations world wide and known by other names.
Lipid organization
Montmorillonite is also known to cause micelles (lipid spheres) to assemble together into vesicles. These are structures that resemble cell membranes on many cells. It can also help nucleotides to assemble into RNA which will end up inside the vesicles. It has been demonstrated that this could have generated highly complex RNA polymers that could reproduce the RNA trapped within the vesicles.[8] This process may have led to the origin of life on Earth.[9] Minerals similar to montmorillonites have been found on Mars too.[10]
See also
| Wikimedia Commons has media related to Montmorillonite. |
- Abiogenesis
- Dispersion (soil)
- Sodification
- Emulsion dispersion
References
- ↑ Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W. and Nichols, Monte C. (ed.). "Montmorillonite". Handbook of Mineralogy (PDF). II (Silica, Silicates). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209716. Retrieved December 5, 2011.
- ↑ 2.0 2.1 Montmorillonite. Mindat.org
- ↑ Montmorillonite. Webmineral
- ↑ Hill, Carol; Paolo Forti (1997). "Deposition and Stability of Silicate Minerals". Cave Minerals of the World (Second ed.). National Speleological Society. p. 177. ISBN 1-879961-07-5.
- ↑ Lloyd, Lawrie (2011). Handbook of Industrial Catalysts. New York: Springer. pp. 181–182. ISBN 978-0387246826.
- ↑ Bhattacharyya, KG; Gupta, SS (2008). "Adsorption of a few heavy". Advances in colloid and interface science 140 (2): 114–31. doi:10.1016/j.cis.2007.12.008. PMID 18319190.
- ↑ Saary, J; Qureshi, R; Palda, V; Dekoven, J; Pratt, M; Skotnicki-Grant, S; Holness, L (2005). "A systematic review of contact dermatitis treatment and prevention". Journal of the American Academy of Dermatology 53 (5): 845. doi:10.1016/j.jaad.2005.04.075. PMID 16243136.
- ↑ Clays May Have Aided Formation of Primordial Cells
- ↑ Clay's matchmaking could have sparked life
- ↑ http://www.theguardian.com/science/across-the-universe/2013/jun/07/nasa-mars
- Papke, Keith G. Montmorillonite, Bentonite and Fuller’s Earth Deposits in Nevada, Nevada Bureau of Mines Bulletin 76, Mackay School of Mines, University of Nevada-Reno, 1970.
- Mineral Galleries
- Mineral web
| ||||||||||||||||||||||
