Monoclonal antibody therapy
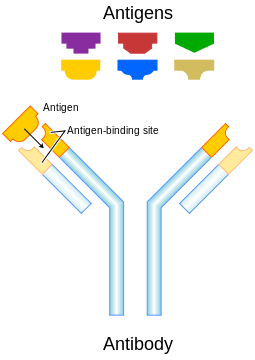
Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to specifically bind to target cells or proteins. This may then stimulate the patient's immune system to attack those cells. It is possible to create a mAb specific to almost any extracellular/ cell surface target, and thus there is a large amount of research and development currently being undertaken to create monoclonals for numerous serious diseases (such as rheumatoid arthritis, multiple sclerosis, Alzheimer's disease, Ebola[1] and different types of cancers). There are a number of ways that mAbs can be used for therapy. For example: mAb therapy can be used to destroy malignant tumor cells and prevent tumor growth by blocking specific cell receptors. Variations also exist within this treatment, e.g. radioimmunotherapy, where a radioactive dose localizes on target cell line, delivering lethal chemical doses to the target.[2]
Structure and function of human and therapeutic antibodies
Immunoglobulin G (IgG) antibodies are large heterodimeric molecules, approximately 150 kDa and are composed of two different kinds of polypeptide chain, called the heavy (~50kDa) and the light chain (~25kDa). There are two types of light chains, kappa (κ) and lambda (λ). By cleavage with enzyme papain, the Fab (fragment-antigen binding) part can be separated from the Fc (fragment constant) part of the molecule (see image). The Fab fragments contain the variable domains, which consist of three antibody hypervariable amino acid domains responsible for the antibody specificity embedded into constant regions. There are four known IgG subclasses all of which are involved in Antibody-dependent cellular cytotoxicity.[3]
The immune system responds to the environmental factors it encounters on the basis of discrimination between self and non-self. Tumor cells are not specifically targeted by one's immune system since tumor cells are the patient's own cells. Tumor cells, however are highly abnormal, and many display unusual antigens that are either inappropriate for the cell type, its environment, or are only normally present during the organisms' development (e.g. fetal antigens).[3]
Other tumor cells display cell surface receptors that are rare or absent on the surfaces of healthy cells, and which are responsible for activating cellular signal transduction pathways that cause the unregulated growth and division of the tumor cell. Examples include ErbB2, a constitutively active cell surface receptor that is produced at abnormally high levels on the surface of approximately 30% of breast cancer tumor cells. Such breast cancer is known a HER2 positive breast cancer.[4]
Antibodies are a key component of the adaptive immune response, playing a central role in both in the recognition of foreign antigens and the stimulation of an immune response to them. The advent of monoclonal antibody technology has made it possible to raise antibodies against specific antigens presented on the surfaces of tumors.[4]
Origins of monoclonal antibody therapy

Immunotherapy developed as a technique with the discovery of the structure of antibodies and the development of hybridoma technology, which provided the first reliable source of monoclonal antibodies, and allowed therapeutic development since the 1970s.[6][7] These advances allowed for the specific targeting of tumors both in vitro and in vivo. Initial research on malignant neoplasms found MAb therapy of limited and generally short-lived success with malignancies of the blood.[8][9] Furthermore treatment had to be specifically tailored to each individual patient, thus proving to be impracticable for the routine clinical setting.
Throughout the progression of monoclonal drug development there have been four major antibody types developed: murine, chimeric, humanised and human.
Initial therapeutic antibodies were simple murine analogues, which contributed to the early lack of success. It has since been shown that these antibodies have: a short half-life in vivo (due to immune complex formation), limited penetration into tumour sites, and that they inadequately recruit host effector functions.[10] To overcome these difficulties the technical issues initially experienced had to be surpassed. Chimeric and humanized antibodies have generally replaced murine antibodies in modern therapeutic antibody applications. Hybridoma technology has been replaced by recombinant DNA technology, transgenic mice and phage display.[11] Understanding of proteomics has proven essential in identifying novel tumour targets.
Murine monoclonal antibodies (suffix -omab)
Initially, murine antibodies were obtained by hybridoma technology, for which Kohler and Milstein received a Nobel prize. However the dissimilarity between murine and human immune systems led to the clinical failure of these antibodies, except in some specific circumstances. Major problems associated with murine antibodies included reduced stimulation of cytotoxicity and the formation complexes after repeated administration, which resulted in mild allergic reactions and sometimes anaphylactic shock.[10]
Chimeric and humanized monoclonal antibodies (suffixes -ximab, -zumab respectively)
To reduce murine antibody immunogenicity, murine molecules were engineered to remove immunogenic content and to increase their immunologic efficiency.[10] This was initially achieved by the production of chimeric and humanized antibodies. Chimeric antibodies are composed of murine variable regions fused onto human constant regions. Human gene sequences, taken from the kappa light chain and the IgG1 heavy chain, results in antibodies that are approximately 65% human. This reduces immunogenicity, and thus increases serum half-life.
Humanised antibodies are produced by grafting murine hypervariable regions on amino acid domains into human antibodies. This results in a molecule of approximately 95% human origin. However it has been shown in several studies that humanised antibodies bind antigen much more weakly than the parent murine monoclonal antibody, with reported decreases in affinity of up to several hundredfold.[12][13] Increases in antibody-antigen binding strength have been achieved by introducing mutations into the complementarity determining regions (CDR),[14] using techniques such as chain-shuffling, randomization of complementarity determining regions and generation of antibody libraries with mutations within the variable regions by error-prone PCR, E. coli mutator strains, and site-specific mutagenesis.[2]
Human monoclonal antibodies (suffix -umab)
Human monoclonal antibodies are produced using transgenic mice or phage display libraries. Human monoclonal antibodies are produced by transferring human immunoglobulin genes into the murine genome, after which the transgenic mouse is vaccinated against the desired antigen, leading to the production of monoclonal antibodies.,[11] allowing the transformation of murine antibodies in vitro into fully human antibodies.[4]
The heavy and light chains of human IgG proteins are expressed in structural polymorphic (allotypic) forms. Human IgG allotype has been considered as one of the many factors that can contribute to immunogenicity.[15] The general scheme of a monoclonal antibody development program is described in.[16]
Targeted conditions
Cancer
Anti-cancer monoclonal antibodies can be targeted against malignant cells by several mechanisms:
- Radioimmunotherapy (RIT) involves the use of radioactively conjugated murine antibodies against cellular antigens. Most research currently involved their application to lymphomas, as these are highly radio-sensitive malignancies. To limit radiation exposure, murine antibodies were especially chosen, as their high immunogenicity promotes rapid clearance from the body. Tositumomab is an example used for non-Hodgkins lymphoma.
- Antibody-directed enzyme prodrug therapy (ADEPT) involves the application of cancer associated monoclonal antibodies which are linked to a drug-activating enzyme. Subsequent systemic administration of a non-toxic agent results in its conversion to a toxic drug, and resulting in a cytotoxic effect which can be targeted at malignant cells. The clinical success of ADEPT treatments has been limited to date.[17] However it holds great promise, and recent reports suggest that it will have a role in future oncological treatment.
- Immunoliposomes are antibody-conjugated liposomes. Liposomes can carry drugs or therapeutic nucleotides and when conjugated with monoclonal antibodies, may be directed against malignant cells. Although this technique is still in its infancy, significant advances have been made. Immunoliposomes have been successfully used in vivo to achieve targeted delivery of tumour-suppressing genes into tumours, using an antibody fragment against the human transferrin receptor. Tissue-specific gene delivery using immunoliposomes has also been achieved in brain, and breast cancer tissue.[18]
Autoimmune diseases
Monoclonal antibodies used for autoimmune diseases include infliximab and adalimumab, which are effective in rheumatoid arthritis, Crohn's disease and ulcerative Colitis by their ability to bind to and inhibit TNF-α.[19] Basiliximab and daclizumab inhibit IL-2 on activated T cells and thereby help preventing acute rejection of kidney transplants.[19] Omalizumab inhibits human immunoglobulin E (IgE) and is useful in moderate-to-severe allergic asthma.
FDA approved therapeutic antibodies
The first FDA-approved therapeutic monoclonal antibody was a murine IgG2a CD3 specific transplant rejection drug, OKT3 (also called muromonab), in 1986. This drug found use in solid organ transplant recipients who became steroid resistant.[20] Hundreds of therapies are undergoing clinical trials. Most are concerned with immunological and oncological targets.
| Antibody | Brand name | Company | Approval date | Type | Target | Indication (Targeted disease) |
|---|---|---|---|---|---|---|
| Abciximab | ReoPro | Eli Lilly | 1994 | chimeric | inhibition of glycoprotein IIb/IIIa | Cardiovascular disease |
| Adalimumab | Humira | Abbott_Laboratories | 2002 | human | inhibition of TNF-α signaling | Several auto-immune disorders |
| Alemtuzumab | Campath | Genzyme | 2001 | humanized | CD52 | Chronic lymphocytic leukemia |
| Basiliximab | Simulect | Novartis | 1998 | chimeric | IL-2Rα receptor (CD25) | Transplant rejection |
| Belimumab | Benlysta | GlaxoSmithKline | 2011 | human | inihibition of B- cell activating factor | Systemic lupus erythematosus |
| Bevacizumab | Avastin | Genentech/Roche | 2004 | humanized | Vascular endothelial growth factor (VEGF) | Colorectal cancer, Age related macular degeneration (off-label) |
| Brentuximab vedotin | Adcetris | 2011 | Chimeric | CD30 | Anaplastic large cell lymphoma (ALCL) and Hodgkin lymphoma | |
| Canakinumab | Ilaris | Novartis | 2009 | Human | IL-1β | Cryopyrin-associated periodic syndrome (CAPS) |
| Cetuximab | Erbitux | Bristol-Myers Squibb/Eli Lilly/Merck KGaA | 2004 | chimeric | epidermal growth factor receptor | Colorectal cancer, Head and neck cancer |
| Certolizumab pegol[21] | Cimzia | UCB (company) | 2008 | humanized | inhibition of TNF-α signaling | Crohn's disease |
| Daclizumab | Zenapax | Genentech/Roche | 1997 | humanized | IL-2Rα receptor (CD25) | Transplant rejection |
| Denosumab | Prolia, Xgeva | Amgen | 2010 | Human | RANK Ligand inhibitor | Postmenopausal osteoporosis, Solid tumor`s bony metasteses |
| Eculizumab | Soliris | Alexion Pharmaceuticals | 2007 | humanized | Complement system protein C5 | Paroxysmal nocturnal hemoglobinuria |
| Efalizumab | Raptiva | Genentech/Merck Serono | 2002 | humanized | CD11a | Psoriasis |
| Gemtuzumab | Mylotarg | Wyeth | 2000 | humanized | CD33 | Acute myelogenous leukemia (with calicheamicin) |
| Golimumab | Simponi | Johnson & Johnson/Merck & Co, Inc. | 2009 | Human | TNF-alpha inihibitor | Rheumatoid arthritis, Psoriatic arthritis, and Ankylosing spondylitis |
| Ibritumomab tiuxetan | Zevalin | Spectrum Pharmaceuticals, Inc. | 2002 | murine | CD20 | Non-Hodgkin lymphoma (with yttrium-90 or indium-111) |
| Infliximab | Remicade | Janssen Biotech, Inc./Merck & Co | 1998 | chimeric | inhibition of TNF-α signaling | Several autoimmune disorders |
| Ipilimumab ( MDX-101 ) | Yervoy | 2011 | Human | blocks CTLA-4 | Melanoma | |
| Muromonab-CD3 | Orthoclone OKT3 | Janssen-Cilag | 1986 | murine | T cell CD3 Receptor | Transplant rejection |
| Natalizumab | Tysabri | Biogen Idec/Élan | 2006 | humanized | alpha-4 (α4) integrin, | Multiple sclerosis and Crohn's disease |
| Ofatumumab | Arzerra | 2009 | Human | CD20 | Chronic lymphocytic leukemia | |
| Omalizumab | Xolair | Genentech/Novartis | 2004 | humanized | immunoglobulin E (IgE) | mainly allergy-related asthma |
| Palivizumab | Synagis | MedImmune | 1998 | humanized | an epitope of the RSV F protein | Respiratory Syncytial Virus |
| Panitumumab | Vectibix | Amgen | 2006 | human | epidermal growth factor receptor | Colorectal cancer |
| Ranibizumab | Lucentis | Genentech/Novartis | 2006 | humanized | Vascular endothelial growth factor A (VEGF-A) | Macular degeneration |
| Rituximab | Rituxan, Mabthera | Biogen Idec/Genentech | 1997 | chimeric | CD20 | Non-Hodgkin lymphoma |
| Tocilizumab ( or Atlizumab ) | Actemra and RoActemra | 2010 | Humanised | Anti- IL-6R | Rheumatoid arthritis | |
| Tositumomab | Bexxar | GlaxoSmithKline | 2003 | murine | CD20 | Non-Hodgkin lymphoma |
| Trastuzumab | Herceptin | Genentech | 1998 | humanized | ErbB2 | Breast cancer |
| Ustekinumab | Stelara | Centocor | 2013 | IL-12, IL-23 | Psoriatic Arthritis, Plaque Psoriasis | |
| Vedolizumab | Entyvio | Takeda | 2014 | humanized | integrin α4β7 | Crohn's disease, ulcerative colitis |
Recently, the bispecific antibodies, a novel class of therapeutic antibodies, have yielded promising results in clinical trials. In April 2009, the bispecific antibody catumaxomab was approved in the European Union.[22][23]
Economics
Since 2000, the therapeutic market for monoclonal antibodies has grown exponentially. The current “big 5” therapeutic antibodies on the market are bevacizumab, trastuzumab (both oncology), adalimumab, infliximab (both autoimmune and inflammatory disorders, ‘AIID’) and rituximab (oncology and AIID) accounted for 80% of revenues in 2006. In 2007, eight of the 20 best-selling biotechnology drugs in the U.S. are therapeutic monoclonal antibodies.[24] This rapid growth in demand for monoclonal antibody production has been well accommodated by the industrialization of mAb manufacturing.[25]
See also
- Antigen 5T4
- Immunotherapy
- Immunoconjugate
- Nomenclature of monoclonal antibodies
- List of monoclonal antibodies
References
- ↑ Gene Garrard Olinger, Jr., James Pettitt, Do Kim, Cara Working, Ognian Bohorov, Barry Bratcher, Ernie Hiatt, Steven D. Hume, Ashley K. Johnson, Josh Morton, Michael Pauly, Kevin J. Whaley, Calli M. Lear, Julia E. Biggins, Corinne Scully, Lisa Hensley, and Larry Zeitlin (2012). "Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques". PNAS 109 (44): 18030–5. doi:10.1073/pnas.1213709109. PMC 3497800. PMID 23071322.
- ↑ 2.0 2.1 2.2 Waldmann, Thomas A. (2003). "Immunotherapy: past, present and future". Nature Medicine 9 (3): 269–277. doi:10.1038/nm0303-269. PMID 12612576.
- ↑ 3.0 3.1 Janeway, Charles; Paul Travers; Mark Walport; Mark Shlomchik (2001). Immunobiology; Fifth Edition. New York and London: Garland Science. ISBN 0-8153-4101-6.
- ↑ 4.0 4.1 4.2 Janeway CA, Jr. et al. (2005). Immunobiology. (6th ed.). Garland Science. ISBN 0-443-07310-4.
- ↑ Modified from Carter P (November 2001). "Improving the efficacy of antibody-based cancer therapies". Nat. Rev. Cancer 1 (2): 118–29. doi:10.1038/35101072. PMID 11905803.
- ↑ Prof FC Breedveld (2000). "Therapeutic monoclonal antibodies". Lancet. doi:10.1016/S0140-6736(00)01034-5.
- ↑ Köhler G, Milstein C (August 1975). "Continuous cultures of fused cells secreting antibody of predefined specificity". Nature 256 (5517): 495–7. Bibcode:1975Natur.256..495K. doi:10.1038/256495a0. PMID 1172191.
- ↑ Nadler LM, Stashenko P, Hardy R et al. (September 1980). "Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen". Cancer Res. 40 (9): 3147–54. PMID 7427932.
- ↑ Ritz J, Schlossman SF (January 1982). "Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma". Blood 59 (1): 1–11. PMID 7032624.
- ↑ 10.0 10.1 10.2 Stern M, Herrmann R (April 2005). "Overview of monoclonal antibodies in cancer therapy: present and promise". Crit. Rev. Oncol. Hematol. 54 (1): 11–29. doi:10.1016/j.critrevonc.2004.10.011. PMID 15780905.
- ↑ 11.0 11.1 Hudson PJ, Souriau C (January 2003). "Engineered antibodies". Nat. Med. 9 (1): 129–34. doi:10.1038/nm0103-129. PMID 12514726.
- ↑ Carter P, Presta L, Gorman CM et al. (May 1992). "Humanization of an anti-p185HER2 antibody for human cancer therapy". Proc. Natl. Acad. Sci. U.S.A. 89 (10): 4285–9. Bibcode:1992PNAS...89.4285C. doi:10.1073/pnas.89.10.4285. PMC 49066. PMID 1350088.
- ↑ Presta LG, Lahr SJ, Shields RL et al. (September 1993). "Humanization of an antibody directed against IgE". J. Immunol. 151 (5): 2623–32. PMID 8360482.
- ↑ Chothia C, Lesk AM, Tramontano A et al. (1989). "Conformations of immunoglobulin hypervariable regions". Nature 342 (6252): 877–83. Bibcode:1989Natur.342..877C. doi:10.1038/342877a0. PMID 2687698.
- ↑ Jefferis, Roy; Marie-Paule Lefranc (July–August 2009). "Human immunoglobulin allotypes". MAbs 1 (4): 332–338. doi:10.4161/mabs.1.4.9122. PMC 2726606. PMID 20073133.
- ↑ Chapman, Kathryn; Nick Pullen, Lee Coney, Maggie Dempster, Laura Andrews, Jeffrey Bajramovic, Paul Baldrick, Lorrene Buckley, Abby Jacobs, Geoff Hale, Colin Green, Ian Ragan and Vicky Robinson (2009). "Preclinical development of monoclonal antibodies". MAbs 1 (5): 505–516. doi:10.4161/mabs.1.5.9676. PMC 2759500. PMID 20065651.
- ↑ Francis RJ, Sharma SK, Springer C et al. (2002). "A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours". Br J Cancer 87 (6): 600–7. doi:10.1038/sj.bjc.6600517. PMC 2364249. PMID 12237768.
- ↑ Krauss WC, Park JW, Kirpotin DB, Hong K, Benz CC (2000). "Emerging antibody-based HER2 (ErbB-2/neu) therapeutics". Breast Dis 11: 113–124. PMID 15687597.
- ↑ 19.0 19.1 Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 241. ISBN 0-443-07145-4.
- ↑ Hooks MA, Wade CS, Millikan WJ (1991). "Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation". Pharmacotherapy 11 (1): 26–37. PMID 1902291.
- ↑ Goel, Niti; Stephens, Sue (2010). "Certolizumab Pegol". MAbs 2 (2): 137–147. doi:10.4161/mabs.2.2.11271. PMC 2840232. PMID 20190560.
- ↑ Chames, Patrick; Baty, Daniel (2009). "Bispecific antibodies for cancer therapy: The light at the end of the tunnel?". MAbs 1 (6): 539–547. doi:10.4161/mabs.1.6.10015. PMC 2791310. PMID 20073127.
- ↑ Linke, Rolf; Klein, Anke and Seimetz, Diane (2010). "Catumaxomab: Clinical development and future directions". MAbs 2 (2): 129–136. doi:10.4161/mabs.2.2.11221.
- ↑ Scolnik, Pablo A. (2009). "mAbs: A business perspective". MAbs 1 (2): 179–184. doi:10.4161/mabs.1.2.7736. PMC 2725420. PMID 20061824.
- ↑ Kelley, Brian (2009). "Industrialization of mAb production technology". MAbs 1 (5): 443–452. doi:10.4161/mabs.1.5.9448. PMC 2759494. PMID 20065641.
External links
- Cancer Management Handbook: Principles of Oncologic Pharmacotherapy (registration required)
| ||||||||||||||||||||||||||||||||||||||||||||||||