Molecular knot
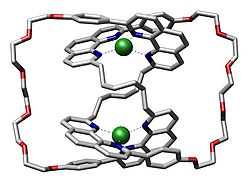
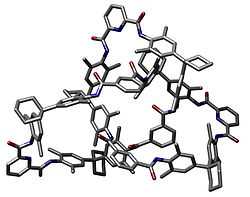
In chemistry, a molecular knot, or knotane, is a mechanically-interlocked molecular architecture that is analogous to a macroscopic knot.[2] A molecular knot in a trefoil knot configuration is chiral, having at least two enantiomers. Examples of naturally formed knotanes are DNA and certain proteins. Lactoferrin has an unusual biochemical reactivity compared to its linear analogue. Other synthetic molecular knots have a distinct globular shape and nanometer sized dimensions that make them potential building blocks in nanotechnology. The first knot was synthesised by Sauvage in 1989 [3]
The term "knotanes" was coined by Fritz Vögtle et al. in Angewandte Chemie International Edition in 2000 by analogy with rotaxane and catenane.[4][5] The term however has yet to be adopted by IUPAC.
Several synthetic knotanes have been reported.[6][7][8][9][10][11] A pentafoil knot has also been reported [12]
See also
References
- ↑ in Recl. Trav. Chim. Pay. B., 1993, 427-428.
- ↑ Lukin, O. and Vögtle, F. (2005), Knotting and Threading of Molecules: Chemistry and Chirality of Molecular Knots and Their Assemblies. Angewandte Chemie International Edition, 44: 1456–1477. doi:10.1002/anie.200460312
- ↑ Dietrich-Buchecker, C. O. and Sauvage, J.-P. (1989), A Synthetic Molecular Trefoil Knot. Angewandte Chemie International Edition in English, 28: 189–192. doi:10.1002/anie.198901891
- ↑ Lukin O, Vögtle F (2005). "Knotting and Threading of Molecules: Chemistry and Chirality of Molecular Knots and Their Assemblies". Angewandte Chemie International Edition 44 (10): 1456–1477. doi:10.1002/anie.200460312. PMID 15704147.
- ↑ Safarowsky O, Nieger M, Fröhlich R, Vögtle F (2000). "A Molecular Knot with Twelve Amide Groups - One-Step Synthesis, Crystal Structure, Chirality". Angewandte Chemie International Edition 39 (9): 1616–1618. doi:10.1002/(SICI)1521-3773(20000502)39:9<1616::AID-ANIE1616>3.0.CO;2-Y. PMID 10820452.
- ↑ Ashton, P. R., Matthews, O. A., Menzer, S., Raymo, F. M., Spencer, N., Stoddart, J. F. and Williams, D. J. (1997), Molecular Meccano, 27. A Template-directed Synthesis of a Molecular Trefoil Knot. Liebigs Annalen, 1997: 2485–2494. doi:10.1002/jlac.199719971210
- ↑ Copper(I)- or Iron(II)-Templated Synthesis of Molecular Knots Containing Two Tetrahedral or Octahedral Coordination Sites, Gwénaël Rapenne, Christiane Dietrich-Buchecker and Jean-Pierre Sauvage, J. Am. Chem. Soc. 1999 121 (5), 994-1001 doi:10.1021/ja982239+
- ↑ Feigel, M., Ladberg, R., Engels, S., Herbst-Irmer, R. and Fröhlich, R. (2006), A Trefoil Knot Made of Amino Acids and Steroids. Angewandte Chemie International Edition, 45: 5698–5702. doi:10.1002/anie.200601111
- ↑ Synthesis of a molecular trefoil knot by folding and closing on an octahedral coordination template Jun Guo, Paul C. Mayers, Gloria A. Breault, Christopher A. Hunter Nature Chemistry 2, 218–222 (2010) doi:10.1038/nchem.544
- ↑ Barran, P. E., Cole, H. L., Goldup, S. M., Leigh, D. A., McGonigal, P. R., Symes, M. D., Wu, J. and Zengerle, M. (2011), Active-Metal Template Synthesis of a Molecular Trefoil Knot. Angewandte Chemie International Edition, 50: 12280–12284. doi:10.1002/anie.201105012
- ↑ Molecular Composite Knots Riccardo F. Carina, Christiane Dietrich-Buchecker, and Jean-Pierre Sauvage Contribution from the Laboratoire de Chimie Organo-Minérale, URA 422 du CNRS, Institut Le Bel, Université Louis Pasteur, 4 rue Blaise Pascal, 67070 Strasbourg-Cedex, France, J. Am. Chem. Soc., 1996, 118 (38), pp 9110–9116 doi:10.1021/ja961459p
- ↑ A synthetic molecular pentafoil knot Jean-François Ayme, Jonathon E. Beves, David A. Leigh, Roy T. McBurney, Kari Rissanen and David Schultz Nature Chemistry 4, 15–20 (2012) doi:10.1038/nchem.1193