Metolachlor
 | |
| Names | |
|---|---|
| IUPAC name
(RS)-2-Chloro-N-(2-ethyl-6-methyl-phenyl)-N-(1-methoxypropan-2-yl)acetamide | |
| Other names
Dual, Pimagram, Bicep, CGA-24705, Pennant. | |
| Identifiers | |
| 51218-45-2 | |
| ChEBI | CHEBI:6902 |
| ChEMBL | ChEMBL1884974 |
| ChemSpider | 4025 |
| |
| Jmol-3D images | Image |
| KEGG | C10953 |
| PubChem | 4169 |
| |
| Properties | |
| Molecular formula |
C15H22ClNO2 |
| Molar mass | 283.79 g·mol−1 |
| Appearance | Off-white to colorless liquid |
| Density | 1.1 g/mL |
| Boiling point | 100 °C (212 °F; 373 K) at 0.001 mmHg |
| 530 ppm at 20 °C | |
| Hazards | |
| Main hazards | [2] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Metolachlor is an organic compound that is widely used as an herbicide. It is a derivative of aniline and is a member of the chloroacetanilide herbicides. It is highly effective toward grasses but its application is also controversial.
Agricultural use
Metolachlor was developed by Ciba-Geigy. Its acts by inhibition of elongases and of the geranylgeranyl pyrophosphate (GGPP) cyclases, which are part of the gibberellin pathway. It is used for grass and broadleaf weed control in corn, soybean, peanuts, sorghum, and cotton. It is also used in combination with other herbicides.
Metolachlor is a popular herbicide in the United States.[3] Metolachlor is becoming less and less common. As originally formulated metolachlor was applied as a racemate, a 1:1 mixture of the (S)- and (R)-stereoisomers. The (R)-enantiomer is inactive, and modern production methods afford only (S)-metolachlor, thus current application rates are far lower than original formulations.
Production and basic structure
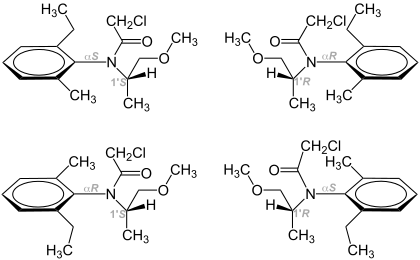
Metolachlor is produced from 2-ethyl-6-methylaniline (MEA) via condensation with methoxyacetone. The resulting imine is hydrogenated to give primarily the S-stereoisomeric amine. This secondary amine is acetylated with chloroacetylchloride. Because of the steric effects of the 2,6-disubstituted aniline, rotation about the aryl-C to N bond is restricted. Thus, for both the (R)- and the (S)-enantiomers exist as atropisomers. Both atropisomers of (S)-metolachlor exhibit the same biological activity.[4]
Safety and ecological effects
Metolachlor has been detected in ground and surface waters in concentrations ranging from 0.08 to 4.5 parts per billion (ppb) throughout the U.S.[5] It is classified as a Category C pesticide by the United States Environmental Protection Agency (US EPA), which indicates limited evidence of carcinogenicity.[6] Evidence of the bioaccumulation of metolachlor in edible species of fish as well as its adverse effect on the growth and development raise concerns on its effects on human health. Though there is no set maximum concentration (maximum contaminant level, MCL) for metolachlor that is allowed in drinking water, the US EPA does have a health advisory level (HAL) of 0.525 mg/L.
Metolachlor induces cytotoxic and genotoxic effects in human lymphocytes.[7] Genotoxic effects have also been observed in tadpoles exposed to metolachlor.[8] Evidence also reveals that metolachlor affects cell growth. Cell division in yeast was reduced,[9] and chicken embryos exposed to metolchlor showed a significant decrease in the average body mass compared to the control.[10]
See also
External Links
- Metolachlor in the Pesticide Properties DataBase (PPDB)
References
- ↑ Extoxnet, Oregon State University
- ↑ Extoxnet Pip - Metolachlor
- ↑ Kiely, T., D. Donaldson, and A. Grube. 2004. Pesticide industry sales and usage: 2000 and 2001 market estimates. US Environmental Protection Agency, Office of Pesticides Programs, Washington, DC
- ↑ H.U.-Blaser (2002). "The Chiral Switch of (S)-Metolachlor: A Personal Account of an Industrial Odyssey in Asymmetric Catalysis". Advanced Synthesis and Catalysis 344: 17–31. doi:10.1002/1615-4169(200201)344:1<17::AID-ADSC17>3.0.CO;2-8.
- ↑ Pothuluri, J.V., Evans, F.E., Doerge, D.R., Churchwell, M.I.,Cerniglia, C.E. (1997). "Metabolism of metolachlor by the fungus Cunninghamella elegans". Arch. Environ. Contam. Toxicol. 32 (2): 117–125. doi:10.1007/s002449900163. PMID 9069185.
- ↑ USEPA,1987. Metolachlor Pesticide Registration Standard. Springfield, IL: Natl. Tech. Info. Serv.
- ↑ Rollof, B., Belluck, D., Meiser, L. (1992). "Cytogenic effects of cyanazine and metolachlor on human lymphocytes exposed in vitro". Mut. Res. Lett. 281 (4): 295–298. doi:10.1016/0165-7992(92)90024-C.
- ↑ Clements, C., Ralph, S.,Petras, M. (1997). "Genotoxicity of select herbicides on Rana catesbeiana tadpoles using alkaline single-cell gel DNA electrophoresis (Comet) assay". Env. Mol. Mut. 29 (3): 277–288. doi:10.1002/(SICI)1098-2280(1997)29:3<277::AID-EM8>3.0.CO;2-9.
- ↑ Echeverrigaray,S., Gomes,L.H., Taveres, F.C.A.(1999). Isolation and characterization of metolachlor resistant mutants of Saccharomyces cerevisiae. World Journal of Micro and Biotech. 15: 679–681.
- ↑ Varnargy,L., Budai, P., Fejes, S., Susan, M., Francsi, T., Keseru, M., Szabo, R.(2003). Toxicity and degradation of metolachlor (Dual 960EC) in chicken embryos. Commun. Agric. Appl. Biol. Sci.68:807–11.
| ||||||||||||||||||||||||||||||||||||||||||