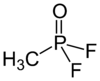Methylphosphonyl difluoride
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methylphosphonic difluoride | |||
| Other names
Methylphosphonyl difluoride Methylphosphonoyl difluoride | |||
| Identifiers | |||
| 4-04-00-03508 | |||
| 676-99-3 | |||
| ChemSpider | 62813 | ||
| |||
| Jmol-3D images | Image | ||
| MeSH | difluoride Methylphosphonic difluoride | ||
| PubChem | 69610 | ||
| |||
| Properties | |||
| CH3POF2 | |||
| Molar mass | 100.00 | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| Infobox references | |||
Methylphosphonyl difluoride (DF), also called methyl difluorophosphite, methylphosphonic difluoride, and difluoromethylphosphine oxide, is a chemical weapon precursor. Its chemical formula is CH3POF2. It is a Schedule 1 substance in the sense of the Chemical Weapons Convention. It is used for production of sarin and soman as a component of binary chemical weapons; an example is the M687 artillery shell, where it is used together with a mixture of isopropyl alcohol and isopropyl amine, producing sarin.
Preparation
Methylphosphonyl difluoride can be prepared by reacting methylphosphonyl dichloride with hydrogen fluoride (HF) or sodium fluoride (NaF).
Safety
Methylphosphonyl difluoride is both reactive and corrosive. It is absorbed through skin and cause burns and mild nerve agent symptoms. It reacts with water producing HF fumes and methylphosphonic acid as a result. It is also capable of corroding glass. It has a boiling point of only 55.4 °C at normal atmospheric pressure, and thus a fairly high vapor pressure at room temperature.