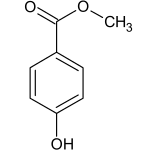
Methylparaben
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methyl 4-hydroxybenzoate | |||
| Other names
Methyl paraben; Methyl p-hydroxybenzoate; Methyl parahydroxybenzoate; Nipagin M; E number E218; Tegosept; Mycocten | |||
| Identifiers | |||
| 99-76-3 | |||
| ChEBI | CHEBI:31835 | ||
| ChEMBL | ChEMBL325372 | ||
| ChemSpider | 7176 | ||
| |||
| Jmol-3D images | Image | ||
| KEGG | D01400 | ||
| PubChem | 7456 | ||
| |||
| UNII | A2I8C7HI9T | ||
| Properties | |||
| Molecular formula |
C8H8O3 | ||
| Molar mass | 152.15 g·mol−1 | ||
| Hazards | |||
| NFPA 704 | |||
| Related compounds | |||
| Related Parabens |
Ethylparaben Propylparaben Butylparaben | ||
| Related compounds |
Methyl salicylate (ortho isomer) | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Methylparaben, also methyl paraben, one of the parabens, is a preservative with the chemical formula CH3(C6H4(OH)COO). It is the methyl ester of p-hydroxybenzoic acid.
Natural occurrences
Methylparaben serves as a pheromone for a variety of insects[1] and is a component of queen mandibular pheromone.
Uses
Methylparaben is an anti-fungal agent often used in a variety of cosmetics and personal-care products. It is also used as a food preservative and has the E number E218.
Methylparaben is commonly used as a fungicide in Drosophila food media. To Drosophila, methylparaben is toxic at higher concentrations, has an estrogenic effect, and slows the growth rate in the larval and pupal stages at lower concentrations.[2]
Safety
There is controversy about whether methylparaben or propylparabens are harmful at concentrations typically used in body care or cosmetics. Methylparaben and propylparaben are considered generally recognized as safe (GRAS) for food and cosmetic antibacterial preservation.[3] Methylparaben is readily metabolized by common soil bacteria, making it completely biodegradable.
Methylparaben is readily absorbed from the gastrointestinal tract or through the skin.[4] It is hydrolyzed to p-hydroxybenzoic acid and rapidly excreted in urine without accumulating in the body.[4] Acute toxicity studies have shown that methylparaben is practically non-toxic by both oral and parenteral administration in animals.[4] In a population with normal skin, methylparaben is practically non-irritating and non-sensitizing; however, allergic reactions to ingested parabens have been reported.[4]
Studies indicate that methylparaben applied on the skin may react with UVB, leading to increased skin aging and DNA damage.[5][6]
References
- ↑ Semiochemical - me-4-hydroxybenzoate, pherobase.com
- ↑ Gu Wei (2009). "Toxicity and Estrogen Effects of Methyl Paraben on Drosophila melanogaster". Food Science 30 (1): 252–254.
- ↑ Parabens, Food and Drug Administration
- ↑ 4.0 4.1 4.2 4.3 Soni MG, Taylor SL, Greenberg NA, Burdock GA (October 2002). "Evaluation of the health aspects of methyl paraben: a review of the published literature". Food Chem. Toxicol. 40 (10): 1335–73. doi:10.1016/S0278-6915(02)00107-2. PMID 12387298.
- ↑ Handa, O; Kokura, S; Adachi, S; Takagi, T; Naito, Y; Tanigawa, T; Yoshida, N; Yoshikawa, T (2006). "Methylparaben potentiates UV-induced damage of skin keratinocytes". Toxicology 227 (1–2): 62–72. doi:10.1016/j.tox.2006.07.018. PMID 16938376.
- ↑ Okamoto, Yoshinori; Hayashi, Tomohiro; Matsunami, Shinpei; Ueda, Koji; Kojima, Nakao (2008). "Combined Activation of Methyl Paraben by Light Irradiation and Esterase Metabolism toward Oxidative DNA Damage". Chemical Research in Toxicology 21 (8): 1594–9. doi:10.1021/tx800066u. PMID 18656963.
External links
- Methylparaben at Hazardous Substances Data Bank
- Methylparaben at Household Products Database
| ||||||||||||||||||||||||||||