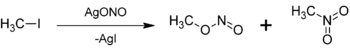Methyl nitrite
 | |
| Identifiers | |
|---|---|
| 624-91-9 | |
| ChemSpider | 11730 |
| |
| Jmol-3D images | Image |
| PubChem | 12231 |
| |
| Properties | |
| Molecular formula |
CH3NO2 |
| Molar mass | 61.04 g·mol−1 |
| Melting point | −16 °C (3 °F; 257 K) |
| Boiling point | −12 °C (10 °F; 261 K) |
| Hazards | |
| MSDS | External MSDS |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
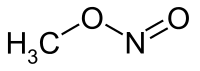
Methyl nitrite is an organic compound with the chemical formula CH
3ONO. It is a gas, and is the simplest alkyl nitrite.
Structure
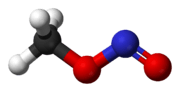
At room temperature, methyl nitrite exists as a mixture of cis and trans conformers. The cis conformer is 3.13 kJ mol−1 more stable than the trans form, with an energy barrier to rotation of 45.3 kJ mol−1.[1]
 |  |
Synthesis
Methyl nitrite can be prepared by the reaction of silver nitrite with iodomethane: Silver nitrite (AgNO2) exists in solution as the silver ion, Ag+ and the nitrite ion, NO2−. One of the lone pairs on an oxygen from nitrite ion attacks the methyl group (—CH3), releasing the iodide ion into solution.[2] Unlike silver nitrite, silver iodide is highly insoluble in water and thus forms a solid.[3] Note that nitrogen is a better nucleophile than oxygen and most nitrites would react via an SN2-like mechanism and the major product would be nitromethane. For example, sodium and potassium nitrite reacting with iodomethane would produce mostly nitromethane, with methyl nitrite as the minor product. However, the presence of the silver ion in solution has a stabilizing effect on the formation of carbocation intermediates, increasing the percent yield of methyl nitrite. In either case, some nitromethane and methyl nitrite are both formed.[2]
Note that this figure contains an error. The left-side product is the methyl nitrite, and the right-side product is supposed to be the nitromethane. The nitro-group in the right-side product has a double bond to both oxygens. This is not correct, as a nitro-group contains a tetravalent and positively charged nitrogen atom with one single bond to the alkyl group, one double bond to an oxygen atom, and one single bond to a second, negatively charged oxygen atom.
This compound is produced by the combustion of unleaded petrol, and might be a cause of the decline of insects, and hence that of the house sparrow and other songbirds in Europe.[4]
See also
References
- ↑ B.J. Van der Veken, R. Maas, G.A. Guirgis, H.D. Stidham, T.G. Sheehan, J.R. Durig (1990). "Infrared spectrum, ab initio calculations, barriers to internal rotation and structural parameters for methyl nitrite". Journal of Physical Chemistry 94 (10): 4029–39. doi:10.1021/j100373a028.
- ↑ 2.0 2.1 Donald L. Pavia, Gary M. Lampman, George S. Kriz (2004). Organic Chemistry 2. Mason, Ohio: Thompson Custom Publishing. ISBN 0-03-014813-8. OCLC 236055357.
- ↑ Darrell D. Ebbing, Steven D. Gammon (2005). General Chemistry (8th ed.). Boston: Houghton Mifflin. ISBN 978-0-618-39941-3.
- ↑ Summers-Smith, J. Denis (September 2007). "Is unleaded petrol a factor in urban House Sparrow decline?". British Birds 100: 558. ISSN 0007-0335.
External links
| ||||||||