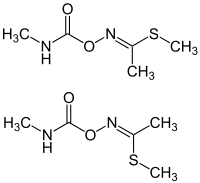
Methomyl
 | |
| Names | |
|---|---|
| IUPAC name
(E,Z)-methyl N-{[(methylamino)carbonyl]oxy}ethanimidothioate | |
| Other names
Lannate, Mesomile, Methomex, Nudrin | |
| Identifiers | |
| 16752-77-5 | |
| ChEBI | CHEBI:6835 |
| ChEMBL | ChEMBL552761 |
| ChemSpider | 3966 |
| |
| Jmol-3D images | Image |
| PubChem | 5353758 |
| |
| Properties | |
| Molecular formula |
C5H10N2O2S |
| Molar mass | 162.21 g·mol−1 |
| Appearance | White crystalline solid[2] |
| Odor | Slight, sulfur-like[2] |
| Density | 1.2946 g/cm3 |
| Melting point | 78 to 79 °C (172 to 174 °F; 351 to 352 K) |
| 58 g/L | |
| Vapor pressure | 0.00005 mmHg (25°C)[2] |
| Hazards | |
| Flash point | Noncombustible[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[2] |
| REL (Recommended) |
TWA 2.5 mg/m3[2] |
| IDLH (Immediate danger) |
N.D.[2] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Methomyl is a carbamate insecticide introduced in 1966. It is highly toxic to humans.[3] The EU and UK imposed a pesticide residue limit of 0.02 mg/kg for apples and oranges.
Methomyl is the active ingredient in commercial fly bait granules which are mixed with a cola drink to kill bothersome raccoon, skunk and opossum. Using methomyl in this "off-label" way may be against the law in some of the United States.[4][5]
Use
Methomyl is an insecticide used against Lepidopterous insect pests, and suppresses Coleopterous and some Hemipterous insect pests. It is also used as an ovicide against cotton bollworms and budworms.[6] Methomyl is registered for commercial/professional use on on a wide variety of agricultural sites including field, vegetable, and orchard crops; turf (sod farms only); and livestock quarters. Its use is also allowed on commercial premises and refuse containers. It is not registered for homeowner or nonprofessional application.[6]
Trade names
Common names for methomyl include metomil and mesomile. Trade names include Acinate, Agrinate, DuPont 1179, Flytek, Kipsin, Lannate, Lanox, Memilene, Methavin, Methomex, Nudrin, NuBait, Pillarmate and SD 14999 [7]
Toxicity
In acute toxicity testing, methomyl is placed in EPA Toxicity Category I (the highest toxicity category out of four) via the oral route and in eye irritation studies.[6] It is in lower Toxicity Categories for inhalation (Category II), acute dermal effects (Category III), and acute skin irritation (Category IV). Methomyl is not likely to be a carcinogen (EPA carcinogen Category E).[6]
Ecotoxicity
Methomyl has low persistence in the soil environment, with a reported half-life of approximately 14 days.[8] Because of its high solubility in water, and low affinity for soil binding methomyl may have potential for groundwater contamination.[6][7] The estimated aqueous half-life for the insecticide is 6 days in surface water and over 25 weeks in groundwater.[7]
Synthesis
Methomyl can be produced by reaction of methyl isocyanate and methylthioacetaldoxime (also known as methomyl oxime).
First prepare ester
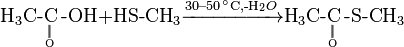
Second prepare oxime from ester
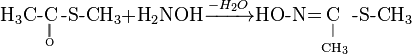
Third prepare product from isocyanate and oxime.
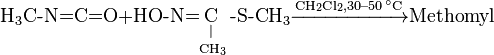
References
- ↑ Merck Index, 11th Edition, 5905
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "NIOSH Pocket Guide to Chemical Hazards #0387". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Methomyl at Extension Toxicology Network
- ↑ Conservation Warden Warns: Fly bait to control wild animals – illegal and a bad idea (Wisconsin Department of Natural Resources)
- ↑ Farm stores promoted poisoning raccoons, state chemist says
- ↑ 6.0 6.1 6.2 6.3 6.4 EPA R.E.D. FACTS - Methomyl (PDF) (Technical report). U. S. Environmental Protection Agency. December 1998. EPA-738-F-98-019.
- ↑ 7.0 7.1 7.2 http://extoxnet.orst.edu/pips/methomyl.htm
- ↑ Howard, P. H. (1991). Handbook of Environmental Fate and Exposure Data for Organic Chemicals: Pesticides. Chelsea, MI: Lewis Publishers. pp. 3–15.
External links
- Methomyl in the Pesticide Properties DataBase (PPDB)
| ||||||||||||||||||||||||||||||||||||||||||||||