Metal–air electrochemical cell
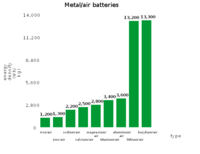
The types of metal–air batteries have different capacities
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous electrolyte.[1][2]
Types
The Li–air battery discharge reaction between Li and oxygen Li2O, according to 4Li + O2 → 2Li2O, has an open-circuit voltage of 2.91 V and a theoretical specific energy of 5210 Wh/kg. Since oxygen is not stored in the battery, the theoretical specific energy excluding oxygen is 11,140 Wh/kg (40.1 MJ/kg). Compare this to the figure of 12,200 Wh/kg (44 MJ/kg) for gasoline (see petrol energy content).
| Metal–air battery | Theoretical specific energy, Wh/kg (including oxygen) |
Theoretical specific energy, Wh/kg (excluding oxygen) |
Calculated open-circuit voltage, V |
|---|---|---|---|
| Aluminium–air | 4300[3] | 8140[4] | 1.2 |
| Beryllium–air | |||
| Calcium-air | 2990 | 4180 | 3.12 |
| Iron–air | |||
| Lithium–air | 5210 | 11140 | 2.91 |
| Magnesium–air | 2789 | 6462 | 2.93 |
| Potassium-air | 935[5][6] | 1700[Note 1] | 2.48[5][6] |
| Sodium–air | 1677 | 2260 | 2.3[7][8] |
| Titanium–air | |||
| Zinc-air | 1090 | 1350 | 1.65 |
See also
References
- ↑ Metal Air Batteries, Half a Fuel Cell?
- ↑ "METAL-AIR BATTERIES Lithium, Aluminum, Zinc, and Carbon" (PDF). Retrieved 2013-04-04.
- ↑ "Electrically Rechargeable Metal-Air Batteries (ERMAB)". Retrieved 25 March 2012.
- ↑ "Batteries for Oxygen Concentrators".
- ↑ 5.0 5.1 "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide".
- ↑ 6.0 6.1 "A Low-Overpotential Potassium−Oxygen Battery Based on Potassium Superoxide".
- ↑ "Electrochemical properties of room temperature sodium–air batteries with non-aqueous electrolyte".
- ↑ "BASF investigating sodium-air batteries as alternative to Li-air; patent application filed with USPTO".
Notes
- ↑ Calculated from the specific energy density (including oxygen) value and 39.1 and 16 atomic weight data for K and O respectively for KO2
| ||||||||||||||||||||
